Essentials of Diagnosis
- Asymmetric hypertrophied nondilated ventricle with septal to posterior wall end-diastolic thickness ≥ 1.3 cm not explained by other etiologies.
- Ejection murmur that increases with Valsalva with or without concomitant mitral regurgitation murmur and preserved aortic second sound.
- Increased gradients causing obstruction across left ventricular outflow tract and/or mid ventricle with characteristic late peaking “dagger”-shaped Doppler velocity profile.
- Mitral systolic anterior motion with varying degree of mitral regurgitation.
- Midsystolic aortic valve closure.
- Impaired diastolic function with decrease in tissue Doppler (early diastolic velocity [E′]) and longitudinal strain.
General Considerations
Hypertrophic cardiomyopathy (HCM) is a disorder of the myocardium caused by mutations of the sarcomere or sarcomere-associated proteins. It mainly manifests as symmetric or asymmetric left ventricular hypertrophy (LVH) > 1.5 cm (Figure 23–1) in a nondilated ventricle unexplained by other cardiac or systemic causes of hypertrophy (see Table 23–1 for differential diagnosis of LVH). HCM has numerous alternate names such as idiopathic hypertrophic subaortic stenosis (IHSS) or asymmetrical septal hypertrophy (ASH). However, HCM is the widely preferred term for this condition as segmental hypertrophy can occur in any segment of the ventricle, not just the septum. Coexistent right ventricular (RV) hypertrophy and RV outflow obstruction occur less commonly in biventricular involvement.
Athlete’s heart Systemic hypertension Subaortic membrane/ridge Aortic stenosis Supravalvular stenosis Right ventricular hypertrophy Fabry disease Glycogen storage disease (PRKAG2 cardiomyopathy, Danon disease, Pompe disease) Mucopolysaccharide storage disease Amyloidosis Sarcoidosis |
HCM is relatively common (1 in 500) in the general population with about 750,000 people affected in the United States. Although it is the most common cause of sudden death in the young (< 35 years old) in North America, most people afflicted with HCM live a normal life. Contrary to the long mistaken notion that HCM was a uniformly fatal disease associated with a shortened life span, HCM patients may live well into their sixth to eighth decades, with patients older than age 90 with HCM being reported. Moreover, the first clinical recognition of HCM may occur when patients are in their sixth to eighth decade of life; usually these patients have milder forms of the disease with the most serious complications being uncommon after age 60. The natural history of HCM can take many paths: sudden cardiac death, symptomatic HCM heart failure, end-stage cardiomyopathy, atrial fibrillation, and stroke. However, if intervened upon in a timely manner, HCM can potentially have no effect on normal longevity.
The genetics of HCM involve an autosomal dominant pattern of inheritance, with 60–70% of patients having an affected family member. HCM is more common in males than females. Common genes affected include β-myosin heavy chain, myosin binding protein C, troponin I, and troponin T, but numerous others have been described. Mutations of the sarcomeric proteins lead to histopathologic evidence of myocardial disarray, which then leads to pathologic hypertrophy and patchy fibrosis of the myocardium. Intramural vessels are also frequently abnormal in HCM with thrombotic obliteration causing ischemia, which may propagate fibrosis regardless of presence of epicardial coronary disease. The genetic basis of ventricular hypertrophy does not always correlate with prognosis. Patients with tropomyosin mutations have only a mild degree of ventricular hypertrophy, with little or no left ventricular (LV) outflow tract obstruction, but they still carry a disproportionately high risk for sudden death.
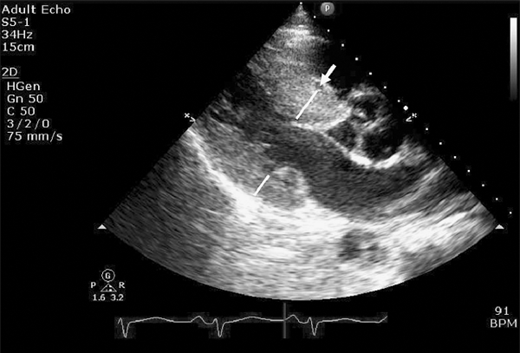
The extent and location of hypertrophy in HCM are highly variable. Asymmetric hypertrophy is most common (septal 90%, midventricular 1%, posteroseptal and lateral wall 1%, apical 3%, symmetric 5%). ASH is defined as septal-to-posterior wall ratio > 1.3 and, in hypertensive patients, > 1.5. Massive hypertrophy > 30 mm is a risk factor for sudden death. Abnormal mitral valve and its apparatus are commonly seen in HCM. Elongated mitral leaflets and abnormal/anteriorly displaced papillary muscles and direct insertion of chordae tendineae or papillary muscles to anterior mitral leaflet can be seen (Figure 23–2). All these factors may contribute to outflow obstruction.
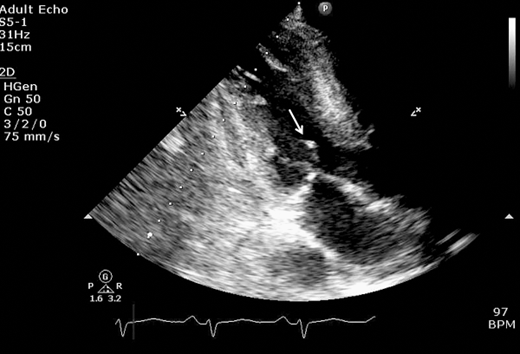
Figure 23–2.
Parasternal long axis view still frame in a patient with obstructive hypertrophic cardiomyopathy. Asymmetric septal hypertrophy (S) is noted along with systolic anterior motion (SAM; blue arrow) and hypertrophied and anteriorly displaced papillary muscle (white arrow) causing further narrowing of left ventricular midcavity and outflow tract.
Pathophysiology
Abnormal diastolic function
Outflow tract obstruction
Mitral regurgitation
Myocardial ischemia
Arrhythmias
Abnormal autonomic function
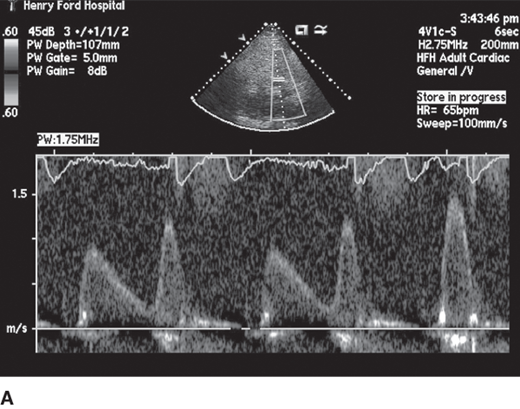
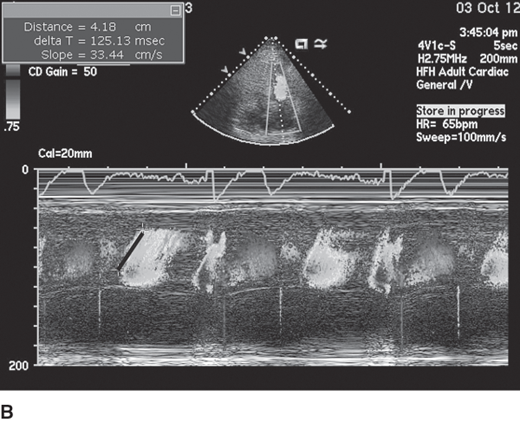
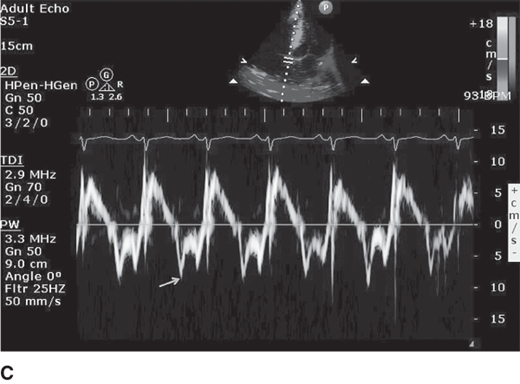
Diastolic filling abnormalities, invariably present in almost all patients with HCM, may precede hypertrophy, and as it worsens, an increased dependence on atrial contribution to ventricular filling occurs. Thus arrhythmias, like atrial fibrillation, are poorly tolerated by patients with HCM and should be promptly treated with sinus rhythm preferentially restored if feasible. Mitral inflow parameters demonstrate varying degrees of dysfunction, most commonly impaired relaxation, whereas restrictive filling patterns are less common but manifest in advanced disease states. Myocardial abnormalities documented by tissue Doppler, speckle tracking echocardiography-based strain, and LV twist and torsion abnormalities have been demonstrated in HCM. Figure 23–3 demonstrates impaired relaxation in mitral inflow, delayed propagation slope on color M-mode, and abnormal tissue Doppler, all indicative of diastolic dysfunction in a patient with HCM.
Figure 23–3.
A: Diastolic mitral inflow profile in a 32-year-old patient with hypertrophic cardiomyopathy (HCM) indicating impaired relaxation pattern. B: Color M-mode flow propagation abnormalities in HCM from same patient. Black slanted line indicates propagation slope of <50 cm/s, indicating impaired relaxation. C: Pulsed tissue Doppler of the septal annulus in a 40-year-old patient with HCM and preserved left ventricular ejection fraction showing low e′ velocities suggesting underlying myocardial dysfunction.
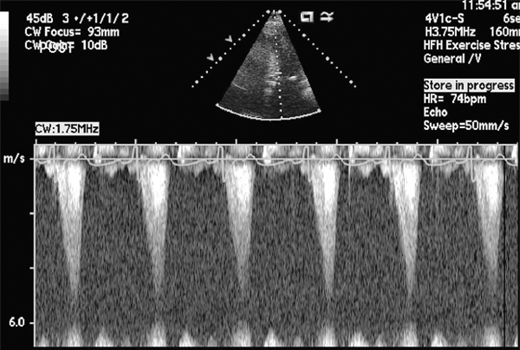
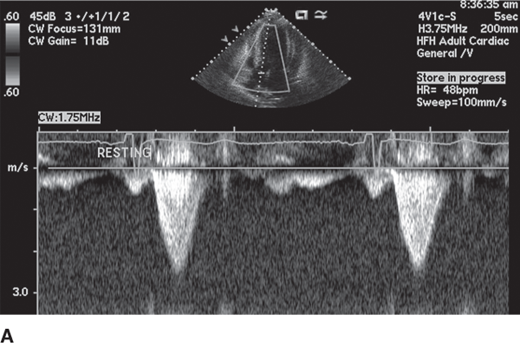
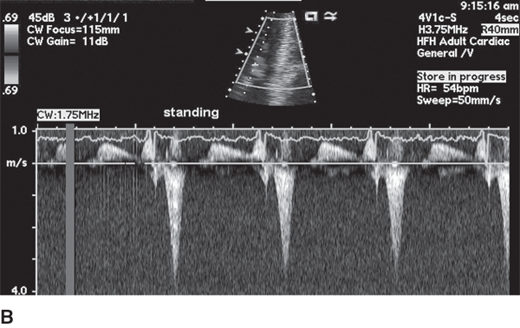
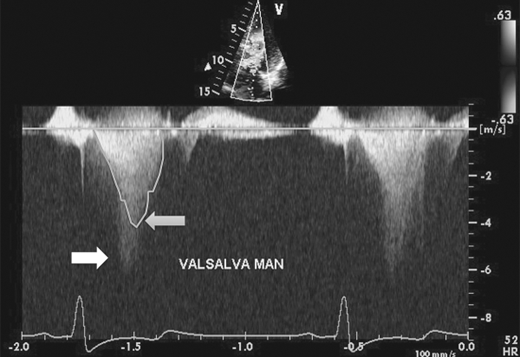
HCM can be broadly categorized into obstructive and nonobstructive types based on presence or absence of gradient (Figure 23–4). About two-thirds of patients with HCM have outflow tract obstruction manifesting as resting or provocable gradient of ≥ 30 mm Hg. However, only 25–30% of patients demonstrate obstruction at rest. Simple maneuvers such as the Valsalva maneuver, standing, or administration of amyl nitrite can provoke or exacerbate outflow tract obstruction and gradients and should be a routine part of clinical and echocardiographic evaluation when HCM is suspected (Table 23–2, Figure 23–5). Outflow obstruction can contribute to exercise intolerance, angina, syncope, and decreased survival. Patients with obstructive HCM are at higher risk of adverse events (heart failure and death) than those without obstruction. The combination of hypertrophied septum bulging into LV outflow tract and abnormal mitral valve motion in systole (systolic anterior motion [SAM]) contribute to outflow obstruction.
Obstruction worsens (gradient increases and murmur increases) 1. Tachycardia 2. Hypovolemia 3. Standing 4. Valsalva 5. Inotropes, diuretics, vasodilators Obstruction lessens (gradient decreases and murmur decreases) 1. Negative inotropes 2. Bradycardia 3. Vasoconstrictors 4. Squatting 5. Isometric handgrip |
Although the exact cause of SAM is debated, two perspectives exist. One is a causal mechanism of SAM precipitated by chordal slackening/anterior displaced mitral valve apparatus leading to “drag forces,” and the other implicates SAM as a consequence of Venturi effect of accelerated flow across the left ventricular outflow tract (LVOT) (Figure 23–6). The severity of obstruction and timing of SAM onset are linearly related. An important caveat is that SAM can be seen in non-HCM states, such as a hyperdynamic small-ventricle, volume-depleted state, post mitral valve repair, and as a nonspecific finding confined to the chordal apparatus (chordal SAM). Thus, although patients in many different pathophysiologic states may exhibit HCM physiology (gradients, SAM), this does not equate to a diagnosis of HCM.
The main etiology of mitral regurgitation (MR) in HCM is malcoaptation of the mitral leaflets secondary to SAM. SAM-related MR is posteriorly directed starting in mid-late systole (Figure 23–7). If a nonposterior jet of MR is seen, then intrinsic mitral valve disease should be suspected. Concomitant primary mitral valve disease is reported in about 20% of patients with HCM (mitral valve prolapse, ruptured chordae, mitral annular calcification, and anomalous or anteriorly displaced papillary muscle) (see Figure 23–2). If SAM is relieved by either myectomy or septal ablation, posterior MR tends to resolve or diminish. Nonposterior MR may require surgical repair or replacement of the valve, which may be one reason why surgical myectomy is favored over ablation as a treatment strategy for HCM.
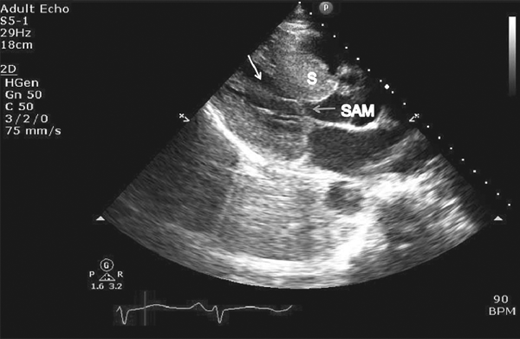
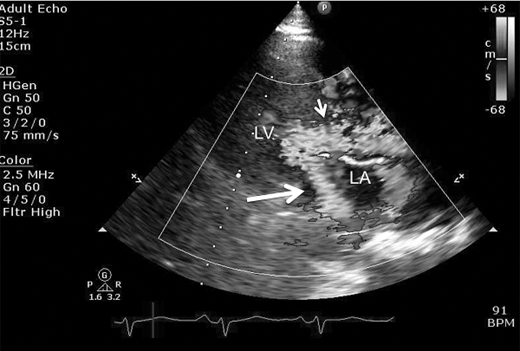
Figure 23–7.
Parasternal long axis view still frame with color illustrating consequence of systolic anterior motion and left ventricular outflow tract (LVOT) obstruction, which leads to turbulent flow across LVOT (small arrow) during left ventricular (LV) systole and posterior directed jet of mitral regurgitation (large thick arrow) into left atrium (LA).
A comprehensive echocardiography assessment of MR is important in HCM. The assessment of peak outflow gradients can be challenging in some patients with HCM in the setting of MR due to contamination of both jets (Figure 23–8). Careful observation of jet profile, coexistent inflow profile, and evaluation of peak gradients from different windows will help to differentiate outflow gradients from MR.
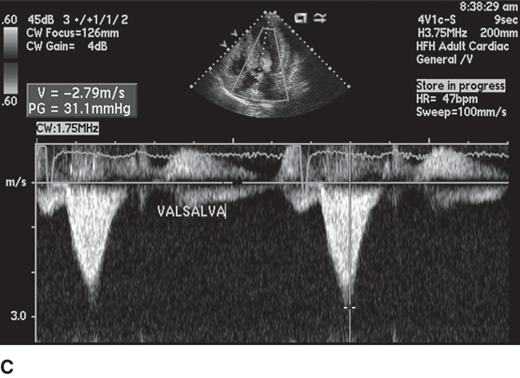
Figure 23–8.
Continuous wave Doppler evaluation of a patient with hypertrophic cardiomyopathy during Valsalva provocation. Two distinct flow patterns are seen: one of the outflow obstruction, which has a late peak (blue arrow), and a superimposed Doppler profile signal of mitral regurgitation (white arrow), making assessment of peak outflow obstruction velocity challenging.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree