Tricuspid Valve Disease
Tricuspid regurgitation
- Prominent v wave in jugular venous pulse.
- Systolic murmur at left lower sternal border that increases with inspiration.
- Characteristic Doppler echocardiographic findings, including right ventricular (RV) volume overload (RV enlargement, paradoxical septal motion, and diastolic flattening of the interventricular septum), right atrial enlargement, and systolic turbulence in the right atrium.
Tricuspid stenosis
- Prominent a wave and reduced y descent in jugular venous pulse.
- Diastolic murmur at left lower sternal border that increases with inspiration.
- Characteristic Doppler echocardiographic findings, including a thickened and domed valve with restricted motion, right atrial enlargement, and increased diastolic velocity across the tricuspid valve.
Clinical interest in tricuspid valve disorders has increased because of several distinct but interrelated events in clinical cardiology. First, high-resolution, noninvasive imaging techniques have been developed and validated, allowing clinicians to easily assess the morphology and function of the tricuspid valve. Second, the frequency of tricuspid valve endocarditis has increased significantly, owing largely to an increasing population of injection drug users, patients with implanted cardiac devices or long-term central venous catheters, and, to a lesser extent, a growing number of immunocompromised patients. Third, several reparative percutaneous and surgical techniques with acceptable morbidity and mortality rates now exist. In addition, investigations in both animals and humans have demonstrated the influence of right-heart disease on cardiovascular performance vis-á-vis series and parallel interactions with the left ventricle.
The tricuspid valve has three leaflets that are unequal in size (anterior > septal > posterior). The papillary muscles are not as well defined as those of the left ventricle and are subject to considerable variation in both their size and leaflet support. Like the mitral valve, the leaflets, annulus, chordae, papillary muscles, and contiguous myocardium contribute individually to normal valve function and can be altered by pathophysiologic processes (Table 21–1).
Tricuspid Regurgitation | Tricuspid Stenosis |
|---|---|
Functional (structurally normal tricuspid valve) | Rheumatic Carcinoid heart disease |
Rheumatic | Tumors |
Infective endocarditis | Congenital (eg, Ebstein anomaly) |
Congenital (eg, tricuspid valve prolapse, Ebstein anomaly) | Regional cardiac tamponade Systemic lupus erythematosus |
Carcinoid heart disease | Whipple disease |
Systemic lupus erythematosus | Fabry disease |
Catheter-induced | Infective endocarditis |
Trauma | Endomyocardial fibrosis |
Tumors | Endocardial fibroelastosis |
Orthotopic heart transplantation | Methysergide therapy |
Endomyocardial fibrosis | Antiphospholipid syndrome |
Antiphospholipid syndrome |
Tricuspid regurgitation most frequently occurs with a structurally normal tricuspid valve (functional tricuspid regurgitation), which is the result of a dilated right ventricle and tricuspid annulus and papillary muscle dysfunction.
Functional tricuspid regurgitation is usually observed in patients with disease of the left heart (eg, left ventricular [LV] dysfunction, mitral valve disease), pulmonary vascular and parenchymal disease, right ventricular (RV) infarction, arrhythmogenic RV dysplasia, and congenital heart disease.
By contrast, organic tricuspid regurgitation occurs when the intrinsic structure of the valve is anatomically abnormal.
Rheumatic tricuspid regurgitation almost always coexists with mitral valve involvement and is due to associated pulmonary hypertension. Although two-thirds of patients with rheumatic mitral valve disease have pathologic evidence of tricuspid valve involvement, clinically significant tricuspid disease, which generally affects young and middle-aged women, is much less common. Rheumatic tricuspid involvement is usually mild and generally is shown clinically as pure regurgitation or mixed regurgitation and stenosis, caused by the fibrosis of the valve leaflets and chordae tendineae; contracture of the leaflets and commissural fusion produce regurgitation and stenosis, respectively.
Tricuspid valve endocarditis occurs primarily in injection drug users and patients with chronic intravascular hardware, left-to-right shunts, burns, and immunocompromised states. Infective endocarditis is more common in injecting drug users who are human immunodeficiency virus (HIV) positive than in those who are HIV negative. Infective endocarditis is typically caused by virulent pathogens that infect structurally normal valves. Staphylococcus aureus is the most common organism; the next most common pathogens are streptococci and enterococci. Pseudomonas and Candida species also predominate, and polymicrobial infections are not uncommon. Geographic location should also be considered when attempting to identify the responsible pathogens. Fungal endocarditis should be considered when vegetations are large; they occasionally cause obstruction. Abscesses may involve the annulus and septum, and chordal rupture or valve perforations may cause tricuspid regurgitation.
Carcinoid tumors are a rare cause of both tricuspid and pulmonic valve disease. The vasoactive substances (principally serotonin) produced by these tumors are believed to be causal, and patients with carcinoid valvular disease have higher serum levels of serotonin and increased urinary excretion of its metabolite, 5-hydroxyindoleacetic acid (5-HIAA), than those without cardiac disease. Left-sided valve involvement is unusual due to inactivation of these vasoactive molecules by monoamine oxidase in the lungs, but can be seen in patients with right-to-left shunts or bronchial tumors. The valve exhibits pathognomonic plaque-like deposits of fibrous tissue (which may also deposit on the endocardium); leaflet distortion leads to regurgitation, stenosis, or both. Tricuspid regurgitation is the most common lesion and is detected by echocardiography in virtually all patients with carcinoid tricuspid valve disease. Cardiac involvement is progressive and causes significant morbidity and mortality in such patients, but early detection and surgical management may prolong survival. Long-term survival has been reported after tricuspid valve replacement, but carcinoid plaques may deposit on the bioprosthetic leaflets.
Tricuspid valve prolapse, owing to myxomatous degeneration, is seen almost exclusively in patients with mitral valve prolapse and occurs in as many as 50% of cases. Isolated involvement has been confirmed, however, by both echocardiography and necropsy. Anterior leaflet prolapse is most common, followed by septal and posterior leaflet prolapse. The associated tricuspid regurgitation is usually mild. Although tricuspid valve prolapse may be a marker of generalized connective tissue disease and a poor prognostic indicator in patients with mitral valve prolapse, its clinical significance remains undefined. Like mitral valve prolapse, the precise incidence of tricuspid valve prolapse is difficult to determine because of inconsistent clinical, echocardiographic, and angiographic definitions.
Tricuspid regurgitation is a frequent component of Ebstein anomaly of the tricuspid valve because of the apical displacement of septal and posterior tricuspid leaflets. This results in “atrialization” of a variable portion of the right ventricle and a range of abnormalities involving the anterior leaflet and atrial septum. The downward displacement of the tricuspid valve frequently causes a tricuspid regurgitant murmur (heard best in the apical area). This uncommon congenital abnormality is associated with right-to-left intra-atrial shunting, RV dysfunction, supraventricular arrhythmias, and sudden death.
Tricuspid regurgitation of at least moderate severity may complicate as many as 25% of cases of systemic lupus erythematosus; significant tricuspid regurgitation is usually due to the pulmonary hypertension produced by lupus pulmonary disease. Libman-Sacks endocarditis that involves the tricuspid valve is far less common. However, Libman-Sacks endocarditis has been associated with antiphospholipid antibodies, which has been shown to cause valvular thickening, isolated tricuspid involvement, and the development of nonbacterial vegetations. The cause of the valve disease is poorly understood, but intravalvular capillary thrombosis is believed to be a factor. Most patients present with combined tricuspid regurgitation and stenosis, and the regurgitation is typically moderate or severe. Pulmonary hypertension is usually present and contributes to the valvular dysfunction.
Although catheter-induced tricuspid regurgitation occurs in approximately 50% of cases with catheters across the valve, the regurgitation is quantitatively small, clinically unimportant, and usually disappears when the catheter is removed. Tricuspid regurgitation can occur as a late complication of successful mitral valve replacement (MVR). In one series, Doppler-detected moderate-to-severe regurgitation occurred in two-thirds of patients at a mean of over 11 years following MVR; over one-third of these patients had clinically evident tricuspid regurgitation. Other causes include blunt and penetrating trauma (rupture of papillary muscles, chordal disruption or detachment, leaflet rupture, complete valve destruction), primary or secondary cardiac tumors, orthotopic heart transplantation, and endomyocardial fibrosis.
Tricuspid stenosis is an uncommon lesion that is usually rheumatic in origin and almost exclusively accompanies mitral stenosis. Isolated rheumatic tricuspid stenosis is rare, and subvalvular disease is usually less severe in tricuspid than in mitral stenosis. Isolated carcinoid tricuspid stenosis is also rare; tricuspid regurgitation is more frequent and often occurs with pulmonic stenosis. Right atrial (RA) myxomas and obstructing metastatic tumors may produce hemodynamic changes that are indistinguishable from tricuspid stenosis. Tricuspid stenosis may be congenital and is infrequently predominant in Ebstein anomaly. Extrinsic compression of the tricuspid valve by a loculated pericardial effusion is an uncommon cause of tricuspid stenosis. Other unusual causes include systemic lupus erythematosus, Whipple disease, Fabry disease, antiphospholipid syndrome, infective endocarditis, endocardial fibroelastosis, and as a sequela to methysergide therapy. It should be noted that prosthetic tricuspid valves, like all prosthetic valves, are inherently stenotic.
Tricuspid valve disease can be difficult to recognize clinically. The symptoms may be overshadowed by associated illness, such as systemic lupus erythematosus, infective endocarditis, trauma, or neoplasia. The dominant presenting features may be symptoms that are usually not considered cardiac in origin: abdominal discomfort, ascites, jaundice, wasting, and inanition. In addition, patients with associated cardiovascular disease may have nonspecific symptoms (exertional dyspnea and fatigue in mitral stenosis) that obfuscate the diagnosis and deflect the suspicion of tricuspid valve disease. In patients with mitral stenosis, for example, tricuspid valve disease protects the pulmonary circulation and exertional dyspnea, pulmonary edema, and hemoptysis are less commonly reported, although a history of excessive fatigue may be elicited. Most often, however, the history is insufficient to diagnose tricuspid valve disease, and only a careful physical examination provides the necessary clues.
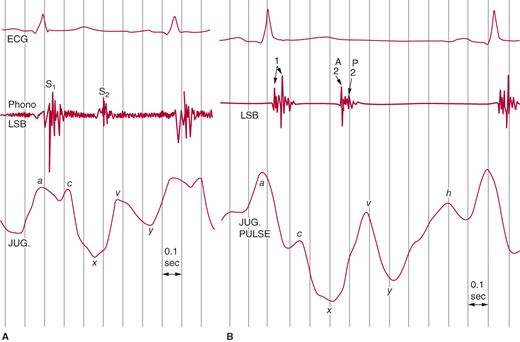
Because the internal jugular veins lack effective valves, they should be inspected for an estimate of RA pressure (Figure 21–1 shows a normal jugular venous pulse). There are three waves (a, c, and v) and two descents, x and y (which correspond to the a and v waves, respectively). The a wave and the initial descent are produced by atrial contraction and relaxation, respectively. The x descent is interrupted by a c wave that is caused by isovolumetric contraction of the right ventricle and resultant bowing of the tricuspid valve toward the right atrium. The continuation of the x descent (sometimes called x′) is caused by the descent of the arteriovenous (AV) ring toward the apex during RV ejection. The right atrium fills and, because the tricuspid valve is closed, RA pressure rises, causing the v wave. The rapid fall in volume and pressure when the valve opens produces the y descent. Except for a small but variable delay, the jugular venous pulse and RA pressure contours are similar.
Figure 21–1.
Normal jugular venous pulse tracings at fast (A) and slow (B) heart rates. The a wave is the dominant reflection. At slow heart rates, an h wave signifying the end of right ventricular filling can be seen. LSB, left sternal border. (Reproduced, with permission, from Tavel ME. Clinical Phonocardiograpy and External Pulse Recoding. Yearbook Medical Publishers, 1978.)
When inspecting the jugular venous pulse, the examiner should pay careful attention to the magnitude of the central venous (mean RA) pressure, the dominant wave (a or v), and changes in pulse contour with respiration. Tricuspid valve disease is typically associated with increased central venous pressure.
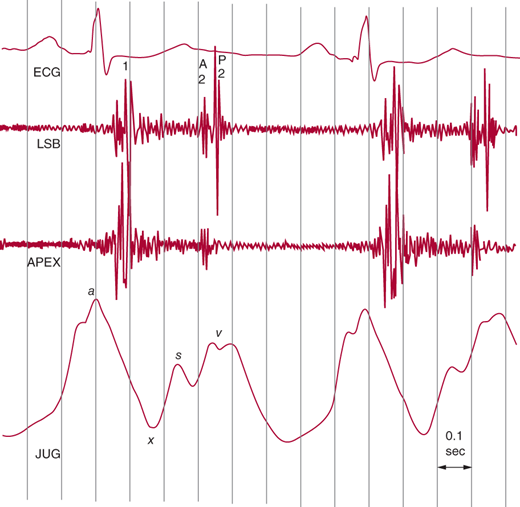
The x descent of the jugular venous pulse is interrupted by an early v wave (c-v wave) with a rapid y descent (Figure 21–2). These findings are characteristically augmented during inspiration. The neck veins are distended, and the earlobes may pulsate. Because venous distention may obscure the jugular pulse contour, it is important to elevate the patient’s head. RV volume overload leads to prominent pulsations over the left lower sternal border.
Figure 21–2.
Jugular venous pulse tracing from a patient with mitral stenosis and tricuspid regurgitation secondary to pulmonary hypertension. Note the shallow x descent, the midsystolic s wave, and the respiratory increase in the v wave. (Reproduced, with permission, from Tavel ME. Clinical Phonocardiography and External Pulse Recording. Yearbook Medical Publishers, 1978.)
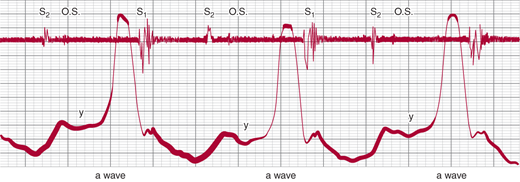
In tricuspid stenosis (Figure 21–3), inspection of the jugular veins reveals a dominant a wave (assuming sinus rhythm), which increases with inspiration, and a slow and shallow y descent due to resistance to early RV diastolic filling.