Congenital Vascular Lesions of the Lungs
Michael J. Liptay
Edward Hong
The incidence of congenital vascular malformations of the lung is low despite the complex embryologic derivation of the pulmonary vasculature (see Chapter 2). Some of the anomalies can be associated with significant disability and still be amenable to appropriate surgical management. A classification of congenital abnormalities that affect the main branches of the pulmonary vessels and their tributaries was suggested by Ellis and associates20 and has been modified here for completeness (Table 85-1).
Agenesis and Stenosis of the Pulmonary Artery
Agenesis and stenosis of pulmonary arteries are rare but provide unusual challenges in diagnosis and management. Most affected persons with these defects die at an early age from right-sided ventricular failure and hypertension in the pulmonary artery of the unaffected side. A few patients are asymptomatic or are hampered by some dyspnea and recurrent pulmonary infections. The natural history of the anomaly is not well understood. Gregg24 reported that these anomalies are associated with maternal rubella.
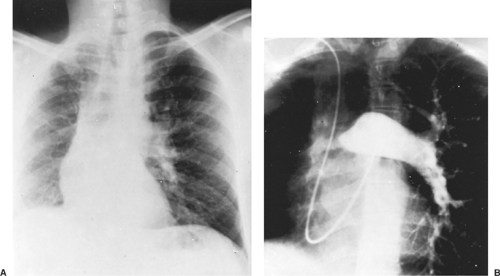
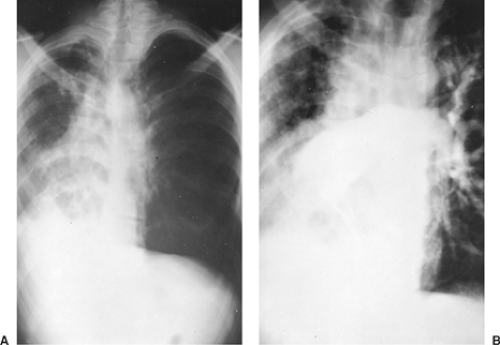
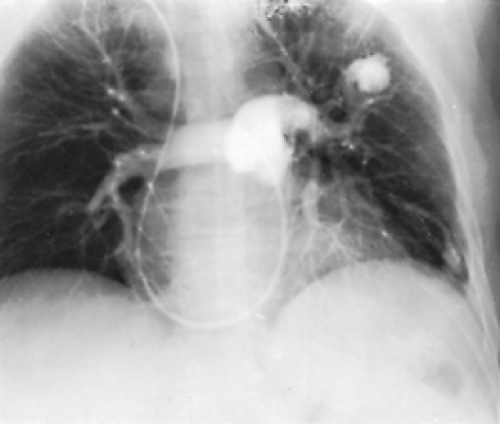
In this abnormality, the affected lung has a small volume and decreased markings (the hyperlucent lung syndrome) (Fig. 85-1). The thoracic cage is contracted and ventilation is reduced. Ventilation/perfusion scans demonstrate vascular hypoperfusion, and decreased ventilation and obstructive bronchiolar disease can be demonstrated with bronchographic contrast material. Angiocardiography delineates the pulmonary artery anomaly. Stenosis of the pulmonary artery may occur at its origin (Fig. 85-2) or at isolated, multiple peripheral sites. In agenesis of the pulmonary artery, an anomalous artery from the aorta (Fig. 85-3) usually supplies the involved lung, but dilated bronchial arteries may be the main source of the blood that maintains some viability of the affected lung. Kochiadakis36 described a patient with an anomalous collateral from a coronary artery that was not associated with coronary ischemia.
Surgical treatment has been reported uncommonly, and although McGoon and Kincaid44 reported repair of multiple peripheral areas of stenosis, most have been at the origin of either mainstem artery. The success of patch arterioplasty or graft replacement of absent or stenosed pulmonary arteries depends largely on the extent of the vascular anomaly and the degree of bronchial and parenchymal lung damage secondary to infection and fibrosis.
Stenoses within the lung parenchyma may be better treated using endovascular or hybrid approaches. Dilatation by balloons has proved to be successful in certain cases of isolated, discrete stenosis of pulmonary arteries. Rocchini and associates55 reported that balloon dilation may be the procedure of choice for multiple stenotic lesions. Successful dilatation, defined as >50% increase in vessel diameter or a decrease in the right ventricular to aortic pressure gradient of ≥50%, occurs in up to 70% of patients. Complications include restenosis, which occurs 20% of the time; transient pulmonary edema; rarely, rupture of the pulmonary artery may be seen. The mortality rate associated with this procedure is 1% to 2%. Cutting balloons consisting of a noncompliant balloon with three or four sharp blades or microtomes bonded longitudinally along the balloon length score the vessel wall, breaking the fibrous and elastic continuity of the lesion. The blades lie within the pleats of the deflated balloon during delivery and retrieval. Recently, these balloons have been used for resistant lesions that fail to respond to
standard or high-pressure balloons, improving success to 80% of patients.17 Boston Children’s Hospital is sponsoring a prospective randomized multicenter trial to evaluate their safety and efficacy.7 Stenting of the affected artery has been described in an effort to decrease rates of restenosis. Large sheaths are needed, which can limit their use to larger patients. In small patients as well in as situations where there is limited vascular access, stent placement in the operating room may be beneficial. Some have argued that reexpansion of the stents as the patient grows can be problematic, but Ungerleider and associates70 found, in a retrospective study, that reexpansion is possible and successful. Antiplatelet therapy is recommended in order to limit restenosis
secondary to intimal proliferation and thrombosis. In older patients with unilateral agenesis of the pulmonary artery, persistent respiratory infection is the most prominent complaint, but the clinical course is often benign and frequently misdiagnosed. Pneumonectomy is usually required, and care to identify and control any systemic artery to the lung is mandatory. Canver and colleagues13 reported the necessity of a pneumonectomy in a neonate with agenesis of the right pulmonary artery who developed an uncontrollable necrotizing bronchopneumonia in the involved lung. This, as these researchers noted, is an unusual complication in the infant or child with this disorder.
standard or high-pressure balloons, improving success to 80% of patients.17 Boston Children’s Hospital is sponsoring a prospective randomized multicenter trial to evaluate their safety and efficacy.7 Stenting of the affected artery has been described in an effort to decrease rates of restenosis. Large sheaths are needed, which can limit their use to larger patients. In small patients as well in as situations where there is limited vascular access, stent placement in the operating room may be beneficial. Some have argued that reexpansion of the stents as the patient grows can be problematic, but Ungerleider and associates70 found, in a retrospective study, that reexpansion is possible and successful. Antiplatelet therapy is recommended in order to limit restenosis
secondary to intimal proliferation and thrombosis. In older patients with unilateral agenesis of the pulmonary artery, persistent respiratory infection is the most prominent complaint, but the clinical course is often benign and frequently misdiagnosed. Pneumonectomy is usually required, and care to identify and control any systemic artery to the lung is mandatory. Canver and colleagues13 reported the necessity of a pneumonectomy in a neonate with agenesis of the right pulmonary artery who developed an uncontrollable necrotizing bronchopneumonia in the involved lung. This, as these researchers noted, is an unusual complication in the infant or child with this disorder.
Table 85-1 Classification of Congenital Vascular Lesions of the Lung | ||
|---|---|---|
|
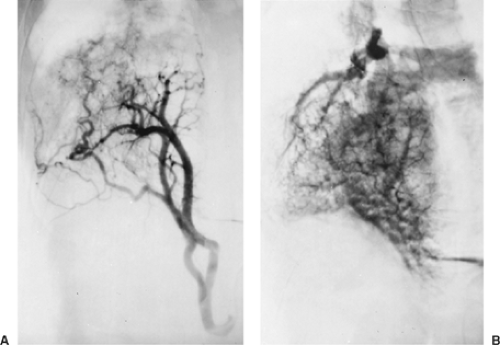 Figure 85-3. This aortogram of the patient shown in Figure 85-2 demonstrates a large ectopic systemic artery that supplies the lower portion of the right lung and arises from the subdiaphragmatic portion of the aorta. B: Delayed radiograph showing venous drainage through normal right pulmonary veins. |
Pulmonary Arteriovenous Fistulas
Pulmonary arteriovenous fistulas (PAVFs) are congenital malformations that result from errant capillary development, with incomplete formation or disintegration of the vascular septa that normally divide the primitive connections between the venous and arterial plexuses. They occur with an incidence of 2 to 3 per 100,000 population and are likely the most common anomaly of the pulmonary vascular tree.33 Tobin67 verified that some pulmonary arteriovenous shunting exists in normal lungs. This shunting may be hemodynamically important in pathologic conditions associated with venous or arterial pulmonary hypertension, portal cirrhosis, and obstructive lung disease. PAVFs interrupt the capillary filter of the lung, allowing emboli that are normally trapped in the pulmonary capillaries to enter the systemic circulation.
Classification
A review of the embryology of the pulmonary vascular system indicates that pulmonary arteriovenous abnormalities may occur as isolated or combined lesions at the arterial, capillary, or venous level. They tend to increase in number and size over time. Anabtawi and colleagues3 presented an anatomic classification based on the size and position of arteriovenous communications (Table 85-2). The classification according to the blood supply has both hemodynamic and prognostic importance because the fistulas supplied by systemic arteries have the same hemodynamic consequences as arteriovenous fistulas of the general circulation. PAVFs usually obtain their afferent supply from one or more branches of the pulmonary artery. However, afferent supply is sometimes partially or completely derived from the systemic circulation: internal mammary, intercostal, or bronchial arteries, or the aorta. PAVFs usually drain into one or more branches of the pulmonary vein, although abnormal efferent vessels may drain directly into the left atrium or inferior vena cava.33 Also, as Dines and associates19 noted,
it is important to divide the entire group into those associated with Rendu-Osler-Weber disease (ROWD), also called hereditary hemorrhagic telangiectasis (HHT), and those not associated with it, because this stratification has important prognostic value. Patients with cutaneous telangiectasis (ROWD) more often have multiple pulmonary fistulas, worse symptoms, rapid disease progression, and a higher rate of complications. Anywhere between 56% and 87% of patients with PAVFs have ROWD, and 20% of patients with ROWD develop fistulas.
it is important to divide the entire group into those associated with Rendu-Osler-Weber disease (ROWD), also called hereditary hemorrhagic telangiectasis (HHT), and those not associated with it, because this stratification has important prognostic value. Patients with cutaneous telangiectasis (ROWD) more often have multiple pulmonary fistulas, worse symptoms, rapid disease progression, and a higher rate of complications. Anywhere between 56% and 87% of patients with PAVFs have ROWD, and 20% of patients with ROWD develop fistulas.
Table 85-2 Classification of Pulmonary Arteriovenous Malformations | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||
Patients with HHT have a high incidence of multiple organs involved with telangiectases: the skin, mucous membranes of the nose, lungs, brain, gastrointestinal tract, and liver. The organ involvement occurs in various combinations, and the sites of involvement appear to be governed by the underlying genetic defect. At present, at least three different phenotypes have been identified. Type I is related to a defect at chromosome 9q33–34, which relates to the protein product endoglin, transforming growth factor-beta (TGF-β) receptor, and mutation and is most often associated with pulmonary arteriovenous malformations. This specific phenotype has been described by Heutink and associates.28 Type II, described by Porteus52 and McAllister43 and their coworkers, is related to chromosome 3q22, which also codes for a TGF-II receptor and is associated, although less frequently, with PAVFs. Type III is related to chromosome 12q and was described by Vincent and colleagues.72 Recently, it was discovered that this gene codes for activin receptorlike kinase 1 which is a type I serine-threonine kinase receptor that can bind TGF-β in the presence of its type II receptor and is expressed on endothelial cells. This latter phenotype does not appear to be associated with pulmonary arteriovenous malformations.
Pulmonary Arteriovenous Fistulas with Pulmonary Arterial Supply
Clinical Aspects
Congenital PAVFs occur more frequently in women and are transmitted as a dominant gene with incomplete penetrance. Hodgson and Kaye29 reported a family that had HHT; 6.4% of those surveyed radiographically had PAVFs. Jeresaty and associates32 reported that symptoms began in childhood and consisted of dyspnea and easy fatigability in 25% to 50% of patients. Most are diagnosed in the third and fourth decades. In patients with HHT, epistaxis is an early sign, and it may begin in adolescence. It is eventually observed in up to 85% of the afflicted patients. Other frequent extrapulmonary symptoms and signs are headaches in 43%, transient ischemic attacks in 57%, and cerebral vascular accidents in 18%, as noted by Goodenberger.23 Less commonly, patients may present with chest pain, cough, tinnitus, vertigo, and ocular symptoms. In the most recent Mayo Clinic review by Swanson and colleagues63 of 93 patients, 84% of patients had symptoms related to PAVF or underlying HHT. Symptoms appear to correlate best with lesion size, with those <2 cm in diameter usually being asymptomatic. Some series have found that patients with multiple rather than single lesions have a higher incidence of symptoms.
Exertional dyspnea, palpitations, and easy fatigability are the most frequent symptoms, usually appearing in the third or fourth decade of life and present in approximately 50% of patients. Hemoptysis is common when the fistula is associated with cutaneous telangiectasis (ROWD). Cutaneous telangiectases are the most common physical finding and are usually located on the face, mouth, chest, and upper extremities. A continuous bruit is present over the lesion in 75% of patients who have associated hereditary telangiectasis and in 38% of those who are without such an association. The typical bruit has a rough, humming, continuous sound that is accentuated in systole and with deep inspiration; this is caused by an increase in pulmonary blood flow. The diastolic accentuation is more subtle. The bruit is decreased by expiration and the Valsalva maneuver, when venous return to the lung is diminished. The audibility of the bruit can change with position. It may be associated with mitral valve prolapse in these patients.
The classic triad of cyanosis, polycythemia, and clubbing of the fingers or toes has been noted in approximately 20% of the patients. The presence of cyanosis indicates that at least 25% to 30% of the blood in the lesser circulation is being shunted from the right to the left side of the heart through the fistula. Platypnea is also occasionally seen and is thought to be related to a decrease in blood flow through the fistula in the dependent portions of the lung while the patient is in the supine position.
Physiologic Findings
The partial pressure of oxygen (PO2) and oxygen saturation are decreased. Swanson and colleagues63 found that the mean PO2 measured on room air was 56 mm Hg. Intracardiac and intravascular pressures proximal to the fistula are normal. As a result, the cardiac output is usually normal, although it may sometimes be increased. The systemic blood pressure and the electrocardiographic findings are also normal. The heart is usually not enlarged. Blood volume study results reveal an increased red cell mass in most cases. The plasma volume remains normal.
Radiographic Features
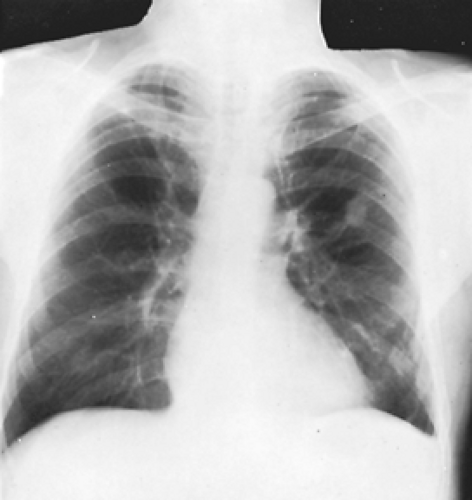
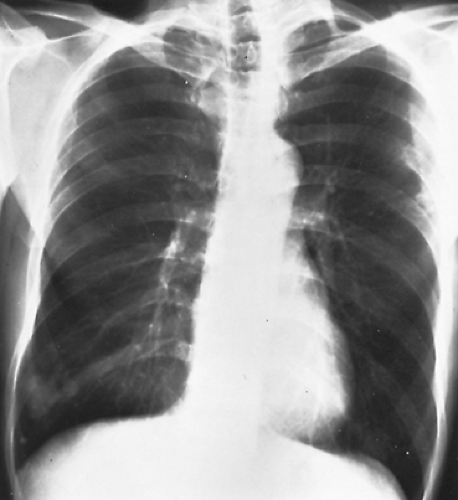
In one-half to two-thirds of the patients, the fistulas are single. The remainder are multiple and may be bilateral in 8% to 10% of patients. Radiographs of the chest have some abnormality 98% of the time and show the fistulas as lobulated, fairly well-defined densities connected to the hilar structures by broad linear shadows because of the dilated vascular connections (Fig. 85-4). Eighty percent of the lesions are subpleural or in the outer third of lung parenchyma. Fistulas are seen more frequently in the lower than in the upper lobes, the left lower lobe being the most common location, followed by the right lower lobe, left upper lobe, right middle lobe, and right upper lobe.33 A solitary lesion initially may be interpreted as a solitary pulmonary nodule, but the characteristic lobulation (the “Mickey Mouse” sign; L. Sider, personal communication, 1993) (Fig. 85-5) should alert one to the true nature of the lesion. On fluoroscopy, these lesions may be seen to pulsate or become smaller with the Valsalva maneuver. Also on fluoroscopy, because of the increased blood flow, there may be an increased amplitude of pulsation of the hilar vessels. Computed tomography (CT) scanning usually demonstrates the lesion sufficiently well to be diagnostic (Fig. 85-6). Remy and associates54 reported that CT scanning enabled identification of 98.2% of 109 PAVFs in 40 patients versus only 59.6% identified by angiography (Fig. 85-7). Angiography, however, is more reliable in the analysis of the angioarchitecture and is a necessary follow-up of the CT scan in those patients who are to undergo interventional management of the fistula.
Helical CT scanning is also an excellent diagnostic tool and may eventually obviate the need for angiography. It is the imaging modality of choice for follow-up after endovascular treatment and for asymptomatic PAVFs smaller than 3 mm.30
Helical CT scanning is also an excellent diagnostic tool and may eventually obviate the need for angiography. It is the imaging modality of choice for follow-up after endovascular treatment and for asymptomatic PAVFs smaller than 3 mm.30
Additional Diagnostic Studies
Burke and Raffin11 suggested the use of noninvasive studies before pulmonary angiography in patients suspected of having PAVFs. These studies include contrast echocardiography, described by Shub and associates,59 and perfusion lung scintigraphy, reported by Lewis and colleagues.38 A negative result excludes the presence of a right-to-left shunt and thus also the presence of a fistula. A positive result confirms the suspected diagnosis without risk to the patient. Contrast echocardiography involves the injection of agitated saline or dye into a peripheral vein. In the presence of a PAVF, bubbles will be visualized in the left atrium after a delay of three to eight cardiac cycles.5 Contrast echocardiography has a sensitivity that approaches 100% but provides no anatomic or quantitative detail of the shunt. Perfusion lung scintigraphy, however, does provide a quantification of shunt magnitude. With the reliability of CT scans, as noted by Remy and associates,54 it would appear that neither of these examinations is necessary, nor would they supplant the use of contrast angiography in patients who are candidates for invasive therapy.
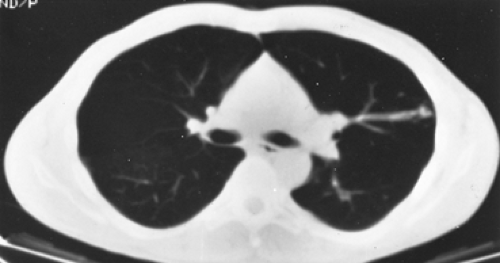 Figure 85-6. Contrast-enhanced CT scan of the same patient in Figure 85-5, showing a different level from the mass seen in the radiograph, reveals several additional arteriovenous malformations in the left lung. The vascular connection to the venous system is clearly demarcated. |
Magnetic resonance imaging (MRI) is another potential modality. There are case reports of successful diagnosis of PAVF by MR angiography (MRA).10 In one such case, a PAVF was detected by gadolinium-enhanced pulmonary MRA and confirmed by pulmonary angiography in a patient whose CT scan was unremarkable.8
Complications
Stringer and associates62 reported 30 instances of serious complications or death among 140 patients with PAVF. Dalton and coworkers16 recorded nine instances of intrapleural rupture. Others, including Iwabuchi and colleagues,31 have noted the occurrence of this potentially fatal complication. Takenaka and associates65 reported the successful resection of the offending fistula in a patient who developed a massive left-sided hemothorax. In addition, these researchers reviewed eight additional successful resections of ruptured fistulas reported in the Japanese literature. Massive hemoptysis after intrabronchial rupture is uncommon. White76 noted that paradoxical embolization and stroke as the result of a bland embolus to the brain may occur. Dines and associates19 observed a stroke to have occurred in 10% of all untreated patients followed for 4 to 10 years in whom a PAVF was seen on the radiograph or if there was hypoxemia on room air. Cerebral abscesses have also been reported, but none of 96 patients reported by Dines and colleagues18 had cerebral abscess or intrapleural rupture. The report of Puskas and colleagues53 noted the occurrence of a brain abscess in several of their patients who were untreated or who had a failed previous balloon occlusion of a pulmonary arteriovenous malformation. Patients with PAVFs should thus have antibiotic prophylaxis for procedures likely to produce bacteremia.29 Yeung and associates82 have observed that neurologic manifestations, especially in those patients with ROWD, occur in 4% to 12% of the cases. In Swanson and colleagues’63 series, neurologic events—including transient ischemic attacks, strokes, brain abscesses, and seizures—were the most prominent complication and occurred in 37% of patients. Strokes were the most common (18%), followed by transient ischemic attacks (12%), abscesses (5%), and seizures (5%).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree