Conduct of Anesthesia
Brian P. Barrick
Robert W. Kyle
Thoracic surgical patients often present with significant comorbidities in addition to compromised pulmonary function. The anesthesiologist, aside from being able to manage such patients, must also acquire a body of knowledge concerning physiology, techniques, and devices unique to the practice of thoracic anesthesia.
Thoracic surgery encompasses a number of procedures involving the airway, bronchial tree, chest wall, mediastinum, and lung parenchyma. This chapter focuses primarily on procedures requiring one-lung ventilation (OLV), such as pulmonary resection. There is also discussion of respiratory physiology, and anesthetic considerations for some of the other procedures performed in thoracic surgery. Last, a discussion of anesthetic management would not be complete without a word on postoperative analgesia.
Characteristics of the Ideal Anesthetic
The thoracic anesthesiologist must have an understanding of airway, thoracic, and bronchial anatomy, pulmonary physiology, gas exchange, airway and lung isolation devices, cardiac–pulmonary interaction, and pain management to meet all of the objectives of providing an anesthetic to the thoracic surgical patient. Some of the characteristics of the ideal anesthetic are explained in more detail later in the chapter.
Hemodynamic (HD) stability during induction of the anesthetic
Use of appropriate devices and techniques to secure the airway and optimize surgical exposure
Maintenance of oxygenation (minimization of shunt)
Maintenance of adequate minute ventilation (elimination of carbon dioxide)
Titration of pharmacologic agents to maintain adequate anesthetic depth, maintain HD stability, and optimize the effects of hypoxic pulmonary vasoconstriction (HPV)
Minimization of barotrauma and air trapping
Optimization of pulmonary vascular resistance and right ventricular function
Application of appropriate agents and techniques to initiate pain control intraoperatively as well as to maintain analgesia and optimization of respiratory function postoperatively
Respiratory Physiology
Some of the physiologic changes that take place with positioning, anesthetic induction, and paralysis are discussed later in the chapter.
Awake, Standing Patient with Spontaneous Respiration
The distribution of ventilation changes with position in the chest. Pleural pressure can be considered “less negative”
(7.5 cm H2O less) as one progresses from the apex to the base of the lung, primarily owing to the effects of gravity.62 This difference causes the apical alveoli to be relatively more distended than the basal alveoli. The basal alveolar units are thus more compliant and distend more per change in unit pressure. The result is distribution of the majority of a ventilation to the basal regions.148
(7.5 cm H2O less) as one progresses from the apex to the base of the lung, primarily owing to the effects of gravity.62 This difference causes the apical alveoli to be relatively more distended than the basal alveoli. The basal alveolar units are thus more compliant and distend more per change in unit pressure. The result is distribution of the majority of a ventilation to the basal regions.148
Distribution of perfusion to the normal lung is also gravity-dependent, with pulmonary vascular pressure decreasing as blood ascends in the chest. West143 divided the lungs into those based on the relationship between alveolar, pulmonary artery, and pulmonary vein pressures. In zone 1 (apical), alveolar pressure exceeds pulmonary vascular pressures and there is relative collapse of vessels. In zones 2 and 3, there is relatively more perfusion as first pulmonary artery pressures and then pulmonary venous pressures exceed alveolar pressures.
So both ventilation and perfusion increase from lung apex to base. What about the ventilation/perfusion ([V with dot above]/[Q with dot above]) ratio? Blood flow increases more rapidly than does ventilation as one moves basally. Therefore apical alveoli are relatively overventilated ([V with dot above]/[Q with dot above]>1) while basal alveoli are overperfused ([V with dot above]/[Q with dot above]<1).78 Note that uneven [V with dot above]/[Q with dot above] relationships in the lungs have a more profound effect on arterial PO2 than PCO2. CO2 is more diffusible and can be eliminated by the overventilated alveoli. However, these same alveoli cannot give up much more oxygen, since the oxyhemoglobin dissociation curve is relatively flat in this region (PO2>90).132
Awake, Lateral Decubitus Position
The vertical gradient of blood flow is the same as in the standing patient, resulting in more blood flow to the dependent (down) lung than to the nondependent (up) lung. This effect is exaggerated when the right lung is down. However, the average effect is for 60% of blood to go to the down lung.150 Gravity has the same effect on pleural pressure and the distribution of ventilation.9 The lower diaphragm is positioned higher in the chest, resulting in a sharper curve and more efficient contraction than the upper diaphragm. Perfusion to the down lung increases more than ventilation, as above. Thus when a standing person assumes a lateral decubitus position, [V with dot above]/[Q with dot above] ratios are not greatly altered.132
Lateral Decubitus, General Anesthesia
While the inhalational induction of general anesthesia does not cause significant changes in the distribution of perfusion, it does cause changes in the distribution of ventilation.
The major change seen is a decrease in volume (and functional residual capacity [FRC]) in both lungs secondary to loss of chest wall tone. The down lung moves down to a lower, flatter portion (less favorable) of the pressure-volume curve and the up lung moves to a lower but steep portion (more favorable).148 The net result is that the majority of ventilation is switched to the up lung.9
Paralysis and controlled ventilation result in even greater maldistribution of ventilation and perfusion for several reasons. With paralysis, the lower diaphragm no longer contributes to ventilation distribution.101 The mediastinum rests on the down lung, and the abdominal contents push the lower diaphragm cephalad. Flexing the operating table puts more pressure on the dependent chest, decreasing the FRC of the down lung even further.
Opening the nondependent chest eliminates the constrictive effect of the chest wall, allowing the up lung to expand even further. The changes outlined above result in an up lung that is very well ventilated but poorly perfused (dead space) and a down lung that is poorly ventilated but well perfused (shunt). This could result in significant hypoxia. However, measures to assist oxygenation, ventilation, and surgical exposure during open chest procedures are discussed below.
One-Lung Ventilation (OLV) and Hypoxic Pulmonary Vasoconstriction
The surgical lung is often not ventilated during thoracic procedures so as to facilitate exposure. The result is that all blood flowing to the up (nonventilated) lung is shunt flow. This is in addition to anatomic shunt and flow in atelectatic areas of the down lung. Both clinical experience and studies show a lower arterial PO2 and higher alveolar–arterial O2 gradient during OLV.75,132 Arterial CO2 is much less affected (as stated above) as long as minute ventilation is maintained.
If there were no compensatory mechanisms to minimize shunt, roughly 40% of the right heart output would go to the nonventilated lung. Fortunately many such mechanisms exist. Gravity increases blood flow to the down lung. Surgical compression of the pulmonary vasculature and ligation of branches of the involved pulmonary artery further reduce shunt. A severely diseased surgical lung may already have restriction of its blood flow, in which case initiation of OLV would not cause as dramatic a change in arterial oxygenation as expected.63,132
By far the mechanism that has the most effect on the pul- monary vasculature is HPV. The normal response of the pulmo- nary precapillary arterioles to atelectasis/hypoxia is vasocon- striction.52 The mechanism behind HPV is not well understood but is thought to involve a balance of local vasoconstricting and vasodilating substances. Mediators that have been studied include voltage-gated potassium channels,87 prostaglandins,90 calcium channels,76 and nitric oxide.152 In the absence of agents that blunt this response, it is possible to reduce the blood flow through the nonventilated lung by up to 50%, meaning that about 20% of right heart output will go to that lung as opposed to 40% without HPV.75 Various anesthetic and vasoactive agents affect HPV. The effects are summarized in Table 22-1 here but are also discussed and referenced later in this chapter.
Indications for One-Lung Ventilation
In general, the indications for lung isolation can be divided into absolute and relative. Absolute indications fall into three categories. The first involves prevention of a disease process whereby one lung contaminates the contralateral lung. The second involves control of ventilation. The objective here is to divert ventilation from diseased lungs in potentially life-threatening situations (listed in Table 22-2). Inadequate ventilation due to
a large air leak or a tension pneumothroax can occur with conventional two-lung ventilation. The third is for unilateral bronchopulmonary lavage, which is used to treat primary alveolar proteinosis.
a large air leak or a tension pneumothroax can occur with conventional two-lung ventilation. The third is for unilateral bronchopulmonary lavage, which is used to treat primary alveolar proteinosis.
Table 22-1 Effects of Various Anesthetic and Vasoactive Agents on Hypoxic Pulmonary Vasoconstriction | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||
All surgical procedures fall under the heading of relative indications for OLV and can be further divided into higher and lower priority. High-priority indications are technically challenging procedures that benefit greatly from a wide exposure of the lung hilum or the length of the thoracic aorta. Another indication is for video-assisted thoracoscopic procedures (VATS). Visualization of the surgical field is nearly impossible if the surgical lung is ventilated. It should be noted, however, that if OLV proves difficult or impossible (because of significant hypoxia or prohibitive airway pressures), consideration should be given to converting to an open technique (where surgical traction can be placed on the lung for exposure) or abandonment of the procedure. Lower-priority indications include surgical procedures that are technically less demanding and more amenable to surgical traction or packing of the operative lung.147 Indications for OLV are summarized in Table 22-2.56
Double-Lumen Endotracheal Tubes
In the early part of the twentieth century, thoracic surgery was performed on a spontaneously breathing patient without an airway device. Surgeries were limited to quick procedures such as empyema drainage and superficial resections because patients would quickly decompensate. Survival rates greatly increased after endotracheal intubation and controlled positive-pressure ventilation became the standard of care. For the intricate, sometimes time-consuming thoracic procedures we see today to become reality, techniques had to evolve that allowed selective ventilation of the nonsurgical lung to maintain a static surgical field, a hemodynamically stable patient, and a contralateral lung free of contaminants.
Bjork and Carlens16 introduced the first practical method to achieve lung separation, later modified by Robertshaw and Carlens.104 Although several devices have since been introduced into practice, the double-lumen endotracheal tube (ETT) can still be considered the mainstay of thoracic surgery. It allows for rapid deflation of the operative lung, is relatively stable once in
proper position, and allows insufflation of oxygen or application of continuous positive airway pressure (CPAP) to the operative field without much difficulty.
proper position, and allows insufflation of oxygen or application of continuous positive airway pressure (CPAP) to the operative field without much difficulty.
Table 22-2 Indications for Lung Isolation/One-Lung Ventilation | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||
The basic left-sided double-lumen ETT (used for most procedures) consists of a bronchial lumen meant to be placed down the left mainstem bronchus and a tracheal lumen that stops short of the carina and can be used to selectively ventilate the right lung. Two cuffs, one in the trachea above the tracheal lumen opening (transparent) and one above the bronchial opening (blue), make lung isolation possible. Several manufacturers have sizes 35, 37, 39, and 41 Fr for adults. Mallinckrodt’s Bronchocath (the brand most familiar to the authors) also comes in sizes 32 and 28 Fr for pediatric patients. Rusch and Sheridan manufacture a 26-Fr ETT as well.
Selection of an appropriate-sized double-lumen ETT is important. The bronchial lumen should have a leak around it with the cuff deflated and require no more than 3 mL of air to produce a seal. An excessively large ETT can damage the tracheobronchial tree. It is now recognized that placement of a double-lumen ETT that is too small can lead to problems as well. Small ETTs are more prone to dislodgement when the patient’s position is changed (supine to lateral decubitus). They require more air in the bronchial cuff to prevent leak, placing more pressure on the bronchial mucosa. There is also more opportunity to advance a small ETT too far, so that a tidal volume meant for two lungs can be delivered to one lung or even a single lobe,116 resulting in barotrauma.
Although it is acknowledged that selection of a double-lumen ETT of proper size is important, there are no uniform guidelines. Many practitioners have satisfactory results with a 37-Fr tube for adult females and a 39-Fr tube for adult males. It is the authors’ bias to reserve 35-Fr ETTs for patients <63 inches tall and 41-Fr ETTs for those >72 inches tall. Attempts to correlate left bronchial width (and hence appropriate ETT size) with readily measured clinical parameters have met with little success. Hannallah and colleagues53 found a weak correlation of age and height with bronchial size in adult males but not in females. They went on to measure left bronchial diameter from computed tomography (CT) scans and made recommendations based on these. Brodsky and colleagues19 based recommendations of double-lumen ETT size on measurements of tracheal diameter. Brodsky and Lemmens18 later noticed a correlation between tracheal width and left bronchial width. Although not listed here, the recommendations of both Brodsky and Hannallah suggest that a 39-Fr ETT is appropriate for many women and a 41-Fr ETT for many men.
Many practitioners still take patient height into consideration in determining ETT size. In a recent commentary, Slinger,117 while recognizing the lack of consensus among thoracic anesthesiologists, put forth recommendations based on height and gender, suggesting that a 37-Fr ETT was best for most women and a 41-Fr for most men. A properly sized left-sided ETT would have a bronchial lumen tip about 1 to 2 mm less than the width of the left mainstem bronchus. This would have to be measured for each individual tube, as this information is not part of the package insert and variations in the manufacturing process can lead to small variations in size.20
Placement of Double-Lumen ETTs
The double-lumen ETT is initially placed by laryngoscopy. In contrast to a single-lumen ETT, a double-lumen tube has a larger circumference and is more rigid. A thin stylet comes with the tube and is placed in the bronchial lumen. By holding the tube so that the bronchial lumen faces anteriorly, the tip can then be bent to resemble the curve of a single-lumen ETT. It is also suggested that a small amount of water-soluble lubricant be placed on the bronchial cuff. With the larynx in view, the tube is placed carefully in the mouth to avoid tearing the tracheal cuff on the teeth. One should be able to view the bronchial cuff as it passes through the vocal cords. Once this occurs, the stylet is removed by an assistant. The tube is then turned 90 degrees counterclockwise and advanced into the trachea until slight resistance is felt. At this point it is assumed that the bronchial lumen is in the left mainstem bronchus. Although a recent study showed that tube placement was more accurate with the stylet left in place as the tube was advanced,78 the authors recommend removing the stylet to decrease the risk of airway trauma.
Proper placement must now be confirmed. One way to do this is by auscultation of the chest. Initially the tracheal cuff is inflated, the tube is attached to the breathing circuit by a special plastic adaptor, and tracheal placement is confirmed as with a single-lumen ETT (chest rise, sustained end-tidal CO2 by capnography, bilateral breath sounds). Once this is accomplished, the bronchial cuff is inflated with no more than 3 mL of air. If the connection between the circuit and the bronchial lumen is clamped, one should hear clear breath sounds on the right but not on the left. Asymmetric chest rise should also be seen on the right. Likewise, when the connection to the tracheal lumen is clamped, breath sounds should be heard and chest rise seen on the left.
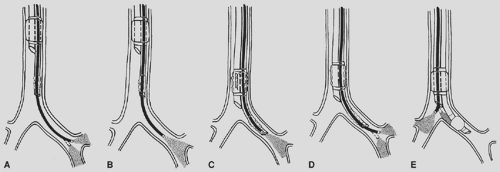
Even if auscultation yields the desired results, tube placement should always be confirmed by fiberoptic bronchoscopy. A standard approach has been described by Slinger119 and is used by the authors. The scope is first passed through the tracheal lumen. The carina should be clearly visualized. For orientation, the anterior and posterior aspects of the trachea can be identified by the presence and absence of rings, respectively. The bronchial lumen should be seen passing down the left mainstem bronchus. In using a BronchoCath tube, a black radiopaque ring on the bronchial lumen (that reflects gray or white with the bronchoscope light) should be seen just above the carina. The superior edge of the inflated bronchial cuff should be barely visible. To confirm that the other bronchus is the right mainstem bronchus, the bifurcation of the right-upper-lobe brochus and the bronchus intermedius should be seen about 1.5 cm from the carina. A look into the right-upper-lobe bronchus should reveal three segmental bronchi. The bronchoscope is then passed down the bronchial lumen to evaluate for tube patency and occlusion of the left lobar bronchi. The left-upper- and lower-lobe bronchi should be seen, with segmental bronchi emerging soon after. Once proper placement is confirmed, the bronchial cuff is deflated until OLV is instituted. Placement must be reconfirmed once the patient is in the lateral decubitus position.
An alternative method of placement has been described by Ovassapian.94 Initially, the tube is placed shallow after laryngoscopy, so that the bronchial lumen’s opening is above the carina. The bronchoscope is inserted down the bronchial lumen and the left mainstem bronchus is inspected. The scope is withdrawn until its tip is just beyond the opening of the bronchial lumen. The tube and scope are then advanced together, and the bronchial lumen can be seen going into the left mainstem bronchus. Alternatively, the scope can remain in the left mainstem bronchus and act as a stylet over which the tube is
advanced into position. The scope is withdrawn and then advanced into the tracheal lumen. The view of the carina, right mainstem bronchus, and tube as described above should be seen. One can then inspect right-sided bronchial anatomy (Fig. 22-1).
advanced into position. The scope is withdrawn and then advanced into the tracheal lumen. The view of the carina, right mainstem bronchus, and tube as described above should be seen. One can then inspect right-sided bronchial anatomy (Fig. 22-1).
Right-Sided Double-Lumen ETTs
Left-sided double-lumen ETTs are preferred over right-sided tubes for the vast majority of procedures requiring lung isolation. The right mainstem bronchus is much shorter because the origin of the right-upper-lobe bronchus is about 1.5 cm from the carina. By contrast, the left mainstem bronchus bifurcates 5 cm from the carina, providing a wider margin of safety12 in placing left-sided tubes. Right-sided tubes are potentially more prone to malposition, leading to right-upper-lobe obstruction (and improper deflation when the right side is the surgical side) and atelectasis (if right-sided ventilation is desired).
There are, however, times when it is difficult or impossible to use a left-sided tube. A classic example is when there is an intraluminal tumor of the left mainstem bronchus. Other situations include an exophytic tumor compressing the left mainstem bronchus or distorting left-sided anatomy, if a left bronchial stent is already in place, or there is left-sided tracheobronchial disruption.22 Other possible indications include unilateral left lung transplantation or left pneumonectomy. A left-sided tube may need to be withdrawn in these situations to prevent interference with an anastomosis or bronchial stapling, resulting in loss of an adequate seal or obstruction of the carina if the bronchial cuff is withdrawn too far.
All right-sided tubes have an additional slot near the distal end of the bronchial lumen to allow ventilation and/or decompression of the right upper lobe. The Mallinckrodt BronchoCath tube, used at the authors’ institution, has a single angled, pear-shaped cuff that encompasses the proximal end of the bronchial slot, allowing a single cuff to provide an adequate seal of both the right upper lobe and the bronchus intermedius. Other manufacturers accomplish this by incorporating two cuffs into the bronchial lumen, one proximal to the bronchial slot and one distal.20
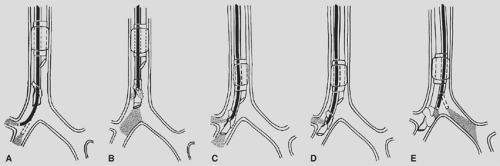
Placement of the right-sided tube is more difficult, and fiberoptic bronchoscopy should be used in the process.25 After intentional shallow placement of the tube in the trachea, the scope is advanced down the right mainstem bronchus. If the space between the carina and right-upper-lobe bronchus is at least 1.5 cm, one can place a right-sided tube.94 One can then either advance the tube over the scope while the right-upper-lobe bronchus is in view or retract the scope until its tip is just distal to the most distal lumen and advance them together into the right main stem. With either technique, one must then be able to view the right upper lobe with its three segmental bronchi through the bronchial slot to ensure proper placement. The scope can then be advanced down the tracheal lumen to inspect the left-sided anatomy and check for bronchial cuff herniation. As always, tube position must be rechecked once the patient is placed in the lateral decubitus position (Fig. 22-2).
Other Lung Isolation Devices
Although double-lumen tubes offer a reliable way to provide absolute lung isolation, they are not ideal for every situation. The most obvious is the patient for whom direct laryngoscopy is difficult or impossible (difficult airway). Double-lumen ETTs are more rigid, have a larger outer circumference, and are longer than their single-lumen counterparts. While there have been reports of successful placement of double-lumen ETTs with awake fiberoptic intubation,96 this is an extremely difficult task even for the most skilled anesthesiologist for the reasons mentioned above. There are a couple of alternatives. One is to fiberoptically intubate with a single-lumen tube and then change to a double-lumen tube with one of several tube exchange devices (mentioned below). Another is to use one of the bronchial blocking devices discussed further on.
There are many instances when a patient will remain ventilated after the procedure. Leaving a double-lumen tube in place for a prolonged period has several disadvantages. The large outer circumference and long bronchial lumen increase the chance of airway damage. The small lumens (less than 5 mm for 39-Fr tubes and smaller) mean that higher peak airway pressures will be needed for adequate ventilation and pulmonary toilet will be difficult. Double-lumen tubes are most frequently changed out for single tubes before transport to the intensive care unit. For critically ill patients who have single-lumen tubes already in place and those for whom the planned procedure will require the administration of a large amount of fluid and blood products (resulting in facial edema and making the airway more difficult to manage), a bronchial blocker will eliminate the need for tube exchange.
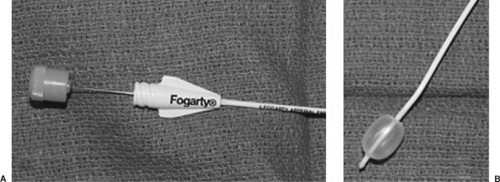
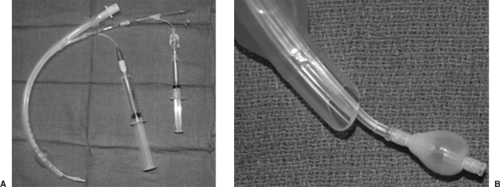
Fogarty Embolectomy Catheter
The Fogarty embolectomy catheter was one of the first devices described for use as an endobronchial blocker.50,137 The most common size used for adults is 8 Fr, with a 14-mL-capacity balloon at the end. The catheter contains a wire stylet, which makes it relatively rigid. The wire, however, does not extend to the distal end of the balloon, so the tip is soft and unlikely to cause bronchial trauma.21 The catheter can be passed through the ETT to the intended bronchus, or direct laryngoscopy can be used to place the catheter through the vocal cords alongside the ETT, so the lumen is not compromised (a consideration for small ETTs). A 30-degree bend at the tip will facilitate placement.94 As with all bronchial blockers, fiberoptic bronchoscopy is recommended to confirm placement into the desired bronchus. If the catheter is to be used within the ETT, an adaptor with an adjustable diaphragm (such as the Arndt multiport adaptor) should be used to allow passage of a fiberoptic scope as well as provide an airtight seal around the catheter, allowing ventilation without significant leak.21
There are several disadvantages to using a Fogarty catheter for lung isolation. Unlike other bronchial blockers, it does not have a hollow lumen that communicates with the lung, so deflation of the surgical lung is strictly by absorption atelectasis and takes a long time. Selective application of CPAP to the surgical lung is also not possible. The balloon is not designed for lung isolation. The spherical low-volume, high-pressure balloon could potentially place a large amount of pressure against the bronchial mucosa, but there are no case reports of such complications. Dislodgement of the catheter is common with changing of patient position or surgical manipulation.94 As with any bronchial blocker, it is recommended that position be confirmed with a fiberoptic scope after changes in patient position (e.g., from supine to lateral) (Fig. 22-3).
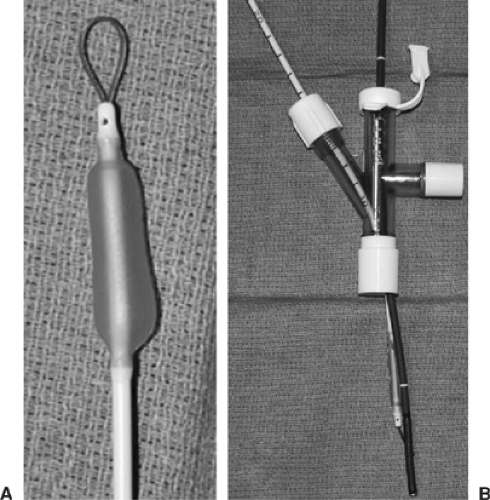
Wire-Guided Endobronchial Blockers
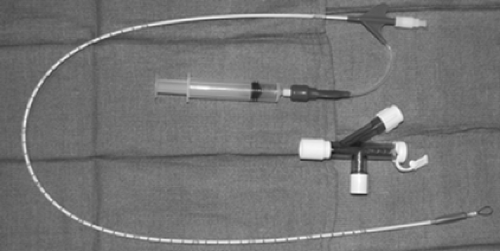
A wire-guided bronchial blocker is used much more commonly in modern anesthetic practice. Arndt developed the first such device in the late 1990s.3 It comes as a 7- or 9-Fr catheter with two lumens: one small (0.4 mm) for inflation of the distal balloon and another larger for lung deflation or application of CPAP. It comes in lengths of 65 cm (7 Fr) and 78 cm (9 Fr), and has depth markings on the surface. There is also a 5-Fr, 50-cm device meant for use in pediatric patients. The original design incorporates an elliptical high-volume, low-pressure balloon that has a greater contact surface area than the Fogarty catheter.3 However, a more spherical design is now available. It has been recommended by Campos21 that the spherical balloon be used in the bronchus intermedius in blocking the right side so as not to include the right-upper-lobe bronchus and to use the elliptical design for occlusion of the left side. If the elliptical balloon is used for right-sided occlusion, right-upper-lobe deflation will have to be achieved before inflation of the balloon for lung isolation. This can be done by manual compression from the surgeon with the
balloon deflated once the chest is open or by applying suction to the right-upper-lobe bronchus through the fiberoptic scope (Figs. 22-4 and 22-5).
balloon deflated once the chest is open or by applying suction to the right-upper-lobe bronchus through the fiberoptic scope (Figs. 22-4 and 22-5).
This device was meant to be placed within a conventional single-lumen ETT. It comes with a multiport adaptor which has three ports: one with an adjustable diaphragm for passage of the blocker to allow an airtight seal around the device, one for passage of the fiberoptic scope, and one that connects to the breathing circuit. It is recommended that at least a 7.0-mm-internal diameter (ID) ETT be used with a 7-Fr catheter and an 8.0-mm-ID tube be used with a 9-Fr catheter,21 keeping in mind that the tube will have to accommodate a 3- to 4-mm scope as well. As the name suggests, there is a flexible nylon wire in the larger lumen, which terminates in a loop at the distal end of the blocker. The loop is meant to accommodate the fiberoptic scope so that, once the scope is in the bronchus to be isolated, the blocker can be advanced over it (the scope acting as a stylet).
Before placement of the blocker, it is recommended that the blocker and fiberoptic scope be placed in the multiport adaptor before the adaptor is attached to the breathing circuit. A small amount of water-soluble lubricant may be placed on the blocker cuff. It is important to deflate the balloon fully and have the blocker port of the adaptor fully open to avoid damage to the balloon. The scope should be passed through the nylon loop before placement of the adaptor on the endotracheal tube. In ventilating the patient during blocker placement, it may be necessary to use high fresh gas flows to compensate for leaks around the scope and blocker. As mentioned above, the scope is introduced into the bronchus to be occluded, then the blocker is advanced over the scope. The wire loop and balloon will be seen past the distal end of the scope when the proper depth is reached. The scope is then retracted above the carina and
5 to 8 mL of air is used to inflate the cuff. The proximal edge of the balloon should be at least 5 mm below the carina in the targeted bronchus. As described above, positioning of the blocker is rechecked after patient position is changed from supine to lateral. One study found the Arndt blocker to be more prone to malposition than other devices (DL ETTs and Univent tubes), but this was not a statistically significant difference—perhaps due to sample size.24
5 to 8 mL of air is used to inflate the cuff. The proximal edge of the balloon should be at least 5 mm below the carina in the targeted bronchus. As described above, positioning of the blocker is rechecked after patient position is changed from supine to lateral. One study found the Arndt blocker to be more prone to malposition than other devices (DL ETTs and Univent tubes), but this was not a statistically significant difference—perhaps due to sample size.24
Once the tube is in proper position, the nylon wire is removed. The larger lumen can now be used to facilitate deflation of the surgical lung, an advantage over the Fogarty catheter. A recent comparison showed that lung collapse took significantly longer with the Arndt blocker than with either DL ETTs or the Univent tube, but that the end result was satisfactory in all groups. It was also noted in this study that, unlike the case with other devices, the majority of patients in whom Arndt devices were used required suction to achieve timely lung collapse.24 The large lumen can also be used to apply CPAP to the surgical lung if the patient experiences profound hypoxia. A connector for a small single-lumen ETT (3.0 mm) can be used to attach it to a CPAP delivery device. One disadvantage is that if the blocker becomes malpositioned after wire removal, repositioning is extremely difficult, since the blocker is flaccid without the wire and the wire cannot be replaced. It has been reported that the Arndt blocker will soon be manufactured with a more rigid wire that can be reinserted.108 The wire of the Fogarty catheter, by contrast, is more rigid and easily replaced. A possible disastrous complication is extrusion of the inflated balloon above the carina and occlusion of the entire airway. Although there are no case reports of this resulting in significant morbidity, it should be suspected if airway pressures rise suddenly during a case and should be acted on immediately. Fiberoptic bronchoscopy should always be used in addressing blocker position.
The Arndt wire-guided bronchial blocker takes longer on average to properly position than other lung isolation devices for both thoracic and nonthoracic anesthesiologists23,24 and, as mentioned above, may be more prone to malpositioning than other lung isolation devices. However, bronchial blocking devices designed for use with single-lumen ETTs provide surgical conditions comparable to other techniques when used properly and have a clear place in the anesthesiologist’s armamentarium. For those patients with difficult airways or anticipated postoperative ventilatory dependence, bronchial blockade offers a safe and effective alternative.
Tip-Deflecting Bronchial Blocker
Recently introduced by Cook Critical Care (Bloomington, IN), the Cohen Tip Deflecting Endobronchial Blocker29 shares many of the characteristics of the Arndt blocker and has the potential to be used in similar situations. The 9-Fr, 65-cm catheter has a low-pressure, high-volume balloon and a central 1.6-mm lumen for lung deflation and CPAP application. The kit comes with an Arndt multiport adaptor for use with a fiberoptic scope. However, the device does not have a distal loop for direct coupling with the scope. It has a soft nylon tip that is coupled with a plastic wheel at the proximal end. The wheel can be turned to deflect the tip as much as 90 degrees, but only in one direction (indicated by an arrow on the distal end of the blocker). In addition, there is a 2-cm external sleeve that can be used to grip the blocker and torque it toward the desired bronchus. Although it is not coupled with a scope, the device should still be placed under direct visualization with the scope placed above the carina. The distal arrow can then be visualized and indicate the direction of deflection of the tip.
This blocker shares many of the advantages of the Arndt blocker. The central lumen allows for lung deflation and CPAP application. One additional advantage is relative ease of repositioning, so that it could be used for sequential lung isolation if needed.108 As with the Arndt, it is recommended that an 8.0-mm-ID ETT be used with the blocker to allow for its manipulation, as well as a 4-mm fiberoptic scope.29 There is one report of the wheel assembly becoming separated from the blocker,40 suggesting that multiple manipulations of the wheel may lead to device failure (Fig. 22-6).
Univent Tube
Introduced in 1982,64 the Univent is a tube with two lumens: a larger lumen designed for ventilation and a smaller one that acts as a channel for an incorporated bronchial blocker. The overall shape is similar to that of a conventional single-lumen ETT. Tube sizes are based on the ID of the ventilatory lumen, and sizes range from 6.0 to 9.0 mm ID. The ventilatory lumen is larger than the blocker channel; it has an oval, almost “D” shape. Thus when we discuss the ID of Univent tubes, this refers to the diameter in the larger dimension. For example, a 8.0 Univent has a ventilatory lumen that is 8 mm by approximately 5 mm. The smaller channel has an incorporated 2-mm movable endobronchial blocker that can be advanced into the desired bronchus or retracted when not in use. This channel increases the anteroposterior (AP) diameter, and it should be noted that Univent tubes have a larger external diameter in general than their single-lumen counterparts. For example, an 8.0 single-lumen ETT has an outer diameter (OD) of 10.8 mm. Contrast this to the 8.0 Univent, which has an OD of 13.5 mm AP and 11.7 mm left to right. The AP diameter would thus be similar to that of a 41-FrDL ETT (Fig. 22-7).20
It has been suggested that, because of its reduced bulk and anatomic angulation, there is less potential for airway trauma with insertion of the Univent compared with a DL ETT.48 While the authors were not able to find studies to support this claim, these characteristics certainly may make this tube easier to insert in a patient with a difficult laryngoscopy. An additional advantage in this regard is that the movable blocker can be advanced prior to insertion and used as a stylet to guide the tube into the trachea, much as one would use an Eschmann introducer.130 However, if a fiberoptic intubation is anticipated or needed after failed intubation attempts, the Univent may be too rigid and bulky to manipulate into the airway over a scope. It would then be the authors’ recommendation to intubate with a conventional single-lumen ETT and use one of the independent bronchial blockers described above.
The blocker has a high-volume, low-pressure cuff that requires 4 to 8 mL of air to achieve occlusion of the mainstem bronchus and about 2 mL for selective lobar occlusion.20 The blocker has a plastic grip at its proximal end (a recent modification)21 to facilitate guidance into the desired bronchus, much like that of the Cohen Flextip blocker. Like other blockers, the device has a hollow lumen that allows for lung deflation either passively or by suctioning. It also allows for the application of CPAP if a connector for a 3.0-mm-ID single-lumen
ETT is placed first.153 The Univent is relatively versatile, with reports of use in patients with tracheostomies8 and to facilitate jet ventilation during sleeve pneumonectomy.146 The blocker is somewhat rigid, so it can be repositioned during a case more readily than a wire-guided blocker. The main proposed advantage of the Univent tube is that, at the end of the case, the bronchial blocker can be retracted and the tube be used for conventional ventilation in those patients with postoperative ventilatory dependence, thus eliminating the need for a change of tubes.
ETT is placed first.153 The Univent is relatively versatile, with reports of use in patients with tracheostomies8 and to facilitate jet ventilation during sleeve pneumonectomy.146 The blocker is somewhat rigid, so it can be repositioned during a case more readily than a wire-guided blocker. The main proposed advantage of the Univent tube is that, at the end of the case, the bronchial blocker can be retracted and the tube be used for conventional ventilation in those patients with postoperative ventilatory dependence, thus eliminating the need for a change of tubes.
Prior to use, the tracheal and bronchial cuffs should be checked for leaks, the bronchial cuff lightly lubricated with a water-soluble lubricant, and the blocker port closed. The Univent can be placed by standard laryngoscopy. The bronchial channel should be facing forward, so that the larger AP diameter passes longitudinally through the vocal cords and reduces the risk of airway trauma. Once through the cords, the channel is turned 90 degrees toward the bronchus to be isolated. A fiberoptic scope is advanced to the distal end of the ventilatory lumen to visualize blocker placement. Placement down the right
main stem may be easier because of its more vertical alignment. As with other bronchial blockers, if the right upper lobe is to be included in the isolation, it may need to be deflated with suction through the scope. Placement down the left main stem may be more difficult, and the ability to torque the blocker with the plastic grip may help with this. Other recommendations include turning the patient’s head to the left while displacing the larynx to the right (although this was described as a blind technique)46 and advancing the blocker channel to just above the left main stem to assist with placement.20 The blocker should be 3 to 5 mm down the desired bronchus and inflated with enough air to prevent a noticeable leak.
main stem may be easier because of its more vertical alignment. As with other bronchial blockers, if the right upper lobe is to be included in the isolation, it may need to be deflated with suction through the scope. Placement down the left main stem may be more difficult, and the ability to torque the blocker with the plastic grip may help with this. Other recommendations include turning the patient’s head to the left while displacing the larynx to the right (although this was described as a blind technique)46 and advancing the blocker channel to just above the left main stem to assist with placement.20 The blocker should be 3 to 5 mm down the desired bronchus and inflated with enough air to prevent a noticeable leak.
In addition to those already mentioned, the Univent has other potential disadvantages. One issue is resistance to airflow when used for conventional ventilation. Recall that the Univent has an oval lumen with a smaller cross-sectional area than that of a single-lumen ETT of the same labeled ID. Since the blocker channel cannot get any smaller, the ventilatory lumen is further compromised as tube size decreases. Indeed, Slinger and colleagues122 showed disproportionately higher resistance to airflow in smaller Univent tubes. It has been recommended that all adults get at least a 7.5-mm Univent and that if a smaller Univent is necessary, that it be exchanged for a single-lumen ETT for postoperative ventilation.20 This eliminates one of the potential advantages of the Univent. It must also be kept in mind that the bronchial blocker can migrate back into the trachea postoperatively and that inadvertent inflation of the bronchial cuff can lead to difficulty with ventilation or respiratory arrest. One such case has been reported.39 If the pilot balloon to the blocker is cut prior to transport to the ICU, this complication can be avoided. Pneumothorax as a complication has been reported,113 but this was felt to be due to the use of a “stylet” technique for insertion of the bronchial blocker, where the entire tube was initially inserted into the left mainstem bronchus, resulting in bronchial trauma. Other complications include detachment of the blocker balloon of a 3.5-mm Univent tube71 and fracture of a sliver of plastic into a patient’s airway (which was recovered) from the proximal end of the tube, possibly because the slip joint of the connector was a bit larger than the ventilatory lumen.37
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree