(1)
Department of Surgery, Robert C. Byrd Health Sciences Center, West Virginia University, 3110 MacCorkle Ave SE, Charleston, WV 25304, USA
(2)
Charleston Area Medical Center, Charleston, WV, USA
(3)
Section of Vascular Surgery and Endovascular Therapy, The University of Chicago, 5841 S. Maryland Ave., MC 5028, Chicago, IL 60637, USA
Abstract
The imaging modalities most often used to evaluate patients for cervical carotid stenosis are carotid duplex ultrasound, computed tomography angiography (CTA), magnetic resonance angiography (MRA), and digital subtraction angiography (DSA).
Duplex ultrasonography provides an accurate noninvasive tool to determine the degree of carotid stenosis and plaque morphology in most patients. It is usually the initial study in patients who present with a carotid bruit or carotid symptoms. This study is highly dependent on technique.
Meanwhile, CTA has recently been regarded as a valuable test for carotid artery stenosis. It is possible to obtain three-dimensional images of the carotid arteries by CTA, although this requires a specialized workstation and dedicated personnel for data processing. CTA cannot be used to evaluate flow dynamics and as such cannot be used for the diagnosis of subclavian steal or other flow-based lesions. The test is easy to perform and associated with few risks. Arterial access is not required, and there is no associated risk of stroke. The image quality rivals that of DSA when the examination is performed on a high-quality helical scanner and reformatted to three-dimensional images by well-trained personnel. CTA can provide additional information about the conformation and composition of the plaque.
CTA is less susceptible than MRA to overestimating the severity of carotid stenosis. It is extremely fast and offers submillimeter spatial resolution, is less expensive than contrast-enhanced MRA, and has the ability to visualize soft tissue, bone, and blood vessels at the same time. CTA can interrogate the arterial tree from the aortic arch to the circle of Willis.
MRA has the advantage of being noninvasive, does not require iodinated contrast or ionizing radiation, and provides unlimited number of projections of the carotid lumen from a single acquisition.
Contrast-enhanced MRA used MR technique to provide flow-independent anatomic information. The technique is somewhat similar to CTA with first-pass MRA. Because these images are not dependent on flow, they provide a more accurate assessment of stenosis and visualization of ulcerated plaques.
Additionally, information about the cerebral circulation can be obtained simultaneously, including patency of the carotid siphon and middle cerebral artery. MRA can also assess intrathoracic and intracranial lesions that are not amenable to duplex interrogation. Using dedicated protocols, MRA can also demonstrate specific plaque components, e.g., calcium, lipid, fibrocellular element, or thrombus within the plaques. The ability to use MRA as a diagnostic tool for carotid stenosis is sadly often dependent on local expertise and familiarity with the test.
This chapter summarizes the role of each imaging modality in the diagnosis of carotid artery disease.
Keywords
CTAMRACarotid diseaseDiagnosisIntroduction
The two most important features of carotid bifurcation atheroma are the degree of diameter stenosis and the character of the carotid bifurcation plaque. There are clinical scenarios where the clinician requires information on the status of the vessels proximal or distal to the cervical carotid artery, in addition to information about the carotid bifurcation. These factors should be considered when choosing between various carotid imaging studies. Some physicians may use multiple modalities when evaluating a patient with suspected cervical carotid stenosis.
In both the North American Symptomatic Carotid Endarterectomy Trial (NASCET) [1, 2] and the European Carotid Surgery Trial (ECST) [3], a higher degree of stenosis in symptomatic patients was associated with a higher stroke risk. In the Asymptomatic Carotid Atherosclerosis Study (ACAS) [4], there was no correlation between the severity of carotid stenosis and the incidence of stroke; however, there were too few strokes in this study to permit a subgroup analysis of the effect of the degree of stenosis on the ability to benefit from carotid endarterectomy. Angiographic data from the ECST study [5], on contralateral asymptomatic carotid arteries, demonstrated a <2% annual stroke risk in patients with less than 70% asymptomatic stenosis. Asymptomatic lesions with greater degrees of stenosis had a greater risk of stroke: 9.8% for patients with 70–79% stenosis and 14.4% for those with 80–99% stenosis. These data suggest that the degree of stenosis is a marker of stroke risk in both symptomatic and asymptomatic lesions. Pathological studies have demonstrated that more stenotic carotid plaques are more likely to have ulceration, intraplaque hemorrhage, and intraluminal thrombus formation, all of which are clearly related to cerebral embolization and stroke [6].
Plaque morphology is an important feature in assessing future risk of neurologic events. This will be discussed in a separate chapter.
Selecting Imaging Modalities for Carotid Evaluation
The imaging modalities most often used to evaluate patients for cervical carotid stenosis are carotid duplex ultrasound, computed tomography angiography (CTA), magnetic resonance angiography (MRA), and digital subtraction angiography.
Carotid Duplex Ultrasound
Duplex ultrasonography provides an accurate noninvasive tool to determine the degree of carotid stenosis and plaque morphology in most patients. It is usually the initial study in patients who present with a carotid bruit or carotid symptoms. The study is highly dependent on technique and should be done in an accredited vascular laboratory (e.g., Intersocietal Commission for the Accreditation of Vascular Laboratories [ICAVL]), and the images should be reviewed by an experienced physician.
Determining the degree of carotid artery stenosis is largely based on an analysis of the peak systolic velocity (PSV) and/or the end-diastolic velocity (EDV) of the carotid artery. A panel of experts from several medical specialties met in October 2002 in San Francisco, California, under the auspices of the Society of Radiologists in Ultrasound to reach a consensus regarding the use of Doppler ultrasound in the diagnosis of internal carotid artery (ICA) stenosis [7]. This panel of experts recommended a cutoff PSV of the ICA of ≥125 and ≥230 cm/s for predicting angiographic >50% and >70% ICA stenoses, respectively. These recommended criteria are based on an analysis of several published studies and the experience of the panelists rather than values validated against other imaging modalities.
AbuRahma et al. [8] recently analyzed the carotid duplex ultrasound and angiography results of 376 carotid arteries in their institution. Using the consensus criteria, they demonstrated a sensitivity of 93%, a specificity of 68%, and an overall accuracy of 85% for stenosis between 50% and 69%. A PSV of ≥230 cm/s for ≥70% stenosis had a sensitivity of 99%, a specificity of 86%, and an overall accuracy of 95%. Receiver operator curves showed that the ICA PSV was significantly better than EDV or ICA/CCA ratio (p = 0.036) in detecting ≥70% stenosis and ≥50% stenosis. There was no improvement in accuracy by adding the EDV values and/or the ratios to the PSV values.
Velocity-based estimation of carotid artery stenosis may need to be adjusted in certain circumstances, e.g., higher velocities in the presence of contralateral carotid artery occlusion and higher velocities in women than in men [9, 10]. Extensive vascular calcification, high carotid bifurcation, severe arterial tortuosity, and obesity may also reduce the accuracy of duplex ultrasound. Carotid stents will decrease compliance of the vessel wall and may increase velocity [11]. Duplex ultrasound may also fail to differentiate between subtotal and total carotid occlusion. Intravenous administration of sonographic contrast agents may improve diagnostic accuracy [12, 13], but the safety of these agents has been questioned.
Contrast ultrasound and power Doppler imaging can be used to differentiate between pre-occlusive stenosis and complete occlusion [14]. Duplex imaging of the carotid artery has two major limitations. These include quality dependence on the technician’s examination and limitations of visualization of the proximal carotid artery and intracranial portions. Although the intracranial cerebral arteries can be assessed with transcranial Doppler, this technique is not as widely available at most institutions as other imaging modalities. Overall, each vascular laboratory should have in place an internal validation process of their own criteria for their internal use.
Computed Tomographic Angiography
Computed tomography angiography, CTA, has only recently been regarded as a valuable test for carotid artery stenosis. While use of CTA for carotid disease is not widespread, CTA is a powerful tool with broad applications.
Most CTA is performed via timing bolus technique. This requires approximately 120 cc of intravenous contrast via a 20-gauge intravenous catheter. Motion and interference artifacts from dental amalgam are the primary source of error in data acquisition. Patient positioning with elevation of the jaw, a shoulder harness, and instructions to avoid swallowing can all be used to eliminate this source of error [15].
While early studies show impressive results regarding CT imaging, analysis of the data is somewhat problematic. Carotid stenosis as evaluated by CTA is described as area reduction of the carotid lumen. This is calculated digitally from images of the entire artery obtained from the scan. This is in distinction from the “gold standard,” digital subtraction angiography, in which diameter reduction is calculated from an estimate of true lumen size [16]. Given that NASCET and ACAS data are based on diameter reduction criteria, it is difficult to interpret CTA data in this regard.
It is possible to obtain three-dimensional images of the carotid arteries by CTA though this requires a specialized workstation and dedicated personnel for data processing. Reformatting of the carotid bifurcation images requires specialized training, a difficult resource to provide for a test that has yet to be widely used by vascular surgeons.
Other disadvantages of CTA include the inability to image the vascular structures of the brain simultaneously. Also, CTA cannot be used to evaluate flow dynamics and as such cannot be used for diagnosis of subclavian steal or other flow-based lesions. Other limitations of this technique include cost (compared to duplex ultrasound), contrast exposure, as well as the added concern of radiation exposure. Additionally, a large calcium burden can limit the ability to distinguish contrast from calcium during post-processing imaging.
While there are several drawbacks to CTA, the advantages are many. The test is easy to perform and associated with few risks. Unlike DSA, arterial access is not required, and there is no associated risk of stroke. The image quality rivals that of DSA when the examination is performed on a high-quality helical scanner with fine cuts and reformatted to three-dimensional images by well-trained personnel (Figs. 15.1 and 15.2).
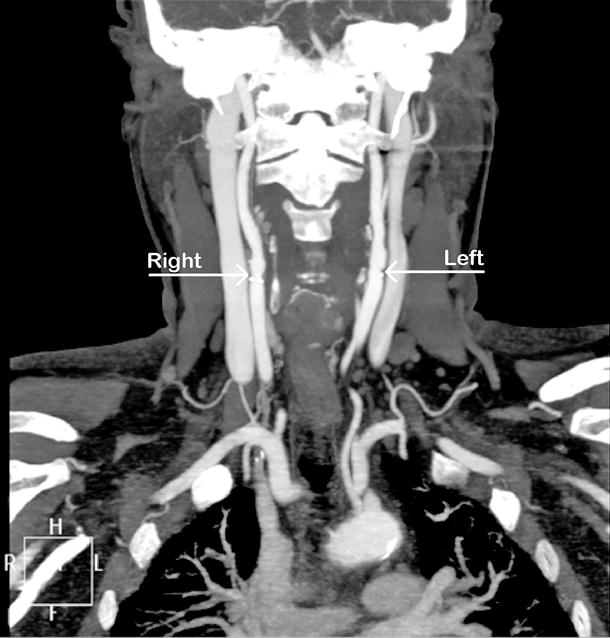
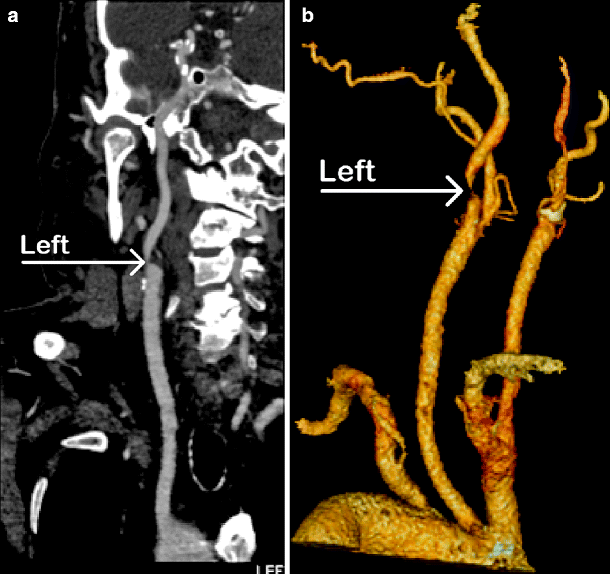

Fig. 15.1
CTA showing minimal carotid disease at right and left bifurcation (arrow)

Fig. 15.2
CTA showing (a) severe left proximal internal carotid artery stenosis (arrow); (b) same patient showing aortic arch vessels (arrow)
More importantly, CTA can provide additional information about the conformation and composition of the plaque, information that may, in the future, be able to distinguish active, dangerous plaque from stable and more benign plaque. Certainly, CTA can “depict plaque morphology, with calcified plaque easily distinguished from soft or lipid laden plaque” [15]. Whether these factors can predict plaque activity and be used to stratify those patients who are at increased risk for stroke is under investigation. Future research focusing on this issue holds many promises and may direct operative therapy more precisely.
CTA is less susceptible than MRA to overestimating the severity of carotid stenosis. The rapid acquisition of spiral CT images allows excellent timing with contrast administration and provides quality images that can be viewed in multiple planes. CTA is extremely fast and offers submillimeter spatial resolution (0.3 vs. 0.8 mm for contrast-enhanced MRA), is less expensive than contrast-enhanced MRA, provides a faster processing time, and has the ability to visualize soft tissue, bone, and blood vessels at the same time. CTA can also demonstrate vascular anomalies, has the ability to quantify the extent of calcification, and can interrogate the arterial tree from the aortic arch to the circle of Willis. Stenoses can be measured with electronic microcalipers based on NASCET or ECST methods [17].
A meta-analysis of 28 studies analyzing the diagnostic accuracy of CTA in comparison to digital subtraction angiography showed a pooled sensitivity of 85% and specificity of 93% for CTA in detecting 70–99% carotid stenosis and a sensitivity and specificity for occlusion of 97% and 99% [18]. CTA was also highly accurate in identifying calcification, but less reliable in describing carotid plaque morphology, specifically the lipid component, or ulceration. CTA appears less reliable than carotid duplex ultrasound or MRA for assessing plaque morphology [19].
Magnetic Resonance Imaging (MRA/MRI)
MRA has the advantage of being noninvasive, does not require iodinated contrast or ionizing radiation, and provides unlimited number of projections of the carotid lumen from a single acquisition. Two techniques are used: time-of-flight imaging (TOF), a flow-dependent technique; and contrast-enhanced MRA (CE MRA), a filling dependent technique, comparable to the technique of CTA. TOF is a widely used technique to establish the diagnosis of carotid stenosis. “This technique is optimized to minimize the signal from stationary tissue, thereby increasing relative signal from the fresh spins delivered to the volume by blood flow from outside the imaging volume” [15]. There are two modes of TOF: two-dimensional and three-dimensional. Two-dimensional time of flight is more sensitive to slower flow, while 3D TOF depicts a wide range of flow velocities and as such has greater accuracy than 2D for defining internal and external carotid artery morphology [15]. Because this imaging is flow dependent, there is some distortion of the carotid anatomy with high-grade lesions or in lesions with turbulent flow. “TOF spins may remain in imaging volume long enough to see numerous pulses and become saturated, thereby causing loss of signal within vessel lumen and inability to depict the vessel contiguous with the lesion” [15]. While this can lead to overestimation of the degree of stenosis and a high false-positive rate, in contrast, the false-negative rate is quite low. Demonstration of minimal disease at the carotid bifurcation on MRA is a highly accurate diagnosis [20].
Contrast-enhanced MRA (CE MRA) uses MR technique to provide flow-independent anatomic information [15]. The technique is somewhat similar to CTA with first-pass MRA. Because these images are not dependent on flow, they provide a more accurate assessment of stenosis and visualization of ulcerated plaques. There may be some technical difficulty capturing the timing bolus; however, once this is overcome, the shorter imaging time increases accuracy secondary to a decreased risk of motion artifact.
The ability to use MRA as a diagnostic tool for carotid stenosis is sadly often dependent on local expertise and familiarity with the test. In centers where the test is widely used, it can provide valuable additional data from that obtained at carotid duplex. MRA has no ionizing radiation or ionic contrast and as such is quite safe for most patients. Additionally, information about the cerebral circulation can be obtained simultaneously including patency of the carotid siphon and MCA.
MRA can also assess intrathoracic and intracranial lesions that are not amenable to duplex interrogation. MRA does not visualize the surrounding soft tissue structures, unless additional magnetic resonance imaging (MRI) is performed and calcium within the plaque is not defined. It cannot be used in patients with implanted ferromagnetic devices (e.g., implantable defibrillators, pacemakers) and is of limited use in uncooperative patients and those with claustrophobia. Gadolinium-based compounds used as a contrast agent for MRA have been associated with nephrogenic systemic fibrosis in patients with preexisting renal disorders [21]. Additionally, small carotid lumens and tortuous vessels can be seen as occlusion or severe stenosis. Finally, the test is quite costly and as such is a less desirable test for screening.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


