Compression of the Trachea by Vascular Rings
Carl L. Backer
The phrase vascular ring refers to a group of congenital vascular anomalies of the aortic arch complex that form an anatomic “ring” that constricts and compresses the trachea or esophagus or both. Hommel, cited by Turner,73 first described a vascular ring, a double aortic arch, in 1737. Almost all clinically significant vascular rings become symptomatic in infants or young children and initially present with airway obstruction from tracheal compression. Esophageal compression and obstruction usually become apparent later, when solid foods are started.
The classification of vascular rings that is used today is based on specific anatomic features, particularly the location of the aortic arch. This classification scheme was endorsed by the Congenital Heart Surgery International Nomenclature and Database Project, as reported by the author and Mavroudis.5 Table 82-1 represents the experience at Children’s Memorial Hospital in Chicago and indicates the relative frequency of the different anomalies that presented for surgical intervention over the past 50 years. Some of these anomalies are anatomically complete rings, or true vascular rings; others are anatomically incomplete, or partial vascular rings, but are grouped with the true vascular rings because they present with similar pathophysiology and hence clinical symptoms. Complete tracheal rings are included because of their close association with pulmonary artery sling, and the similar respiratory symptoms.
Table 82-1 Classification of Vascular Rings and Children’s Memorial Hospital Experience, 1947 to 2006 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||
Gross42 reported the first successful operation for a vascular ring when he divided a double aortic arch that had caused tracheal obstruction in a 1-year-old infant. Gross and Neuhauser44 also reported the first successful suspension of the innominate artery to the sternum for innominate artery compression syndrome in a 4-month-old infant with wheezing and respiratory distress.
Potts and colleagues69 coined the term pulmonary artery sling in their report of successful repair of that anomaly in a 5-month-old with intermittent attacks of dyspnea and cyanosis. Idriss and colleagues48 first reported the successful use of pericardium as a tracheoplasty technique in a 7-month-old with complete tracheal rings. These historical milestones are summarized in Table 82-2.
Embryology
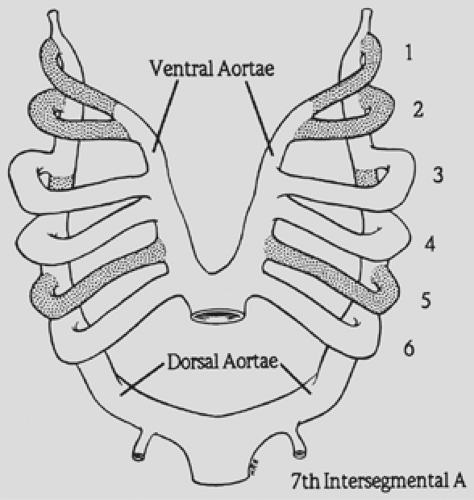
Congdon27 reported an extensive experience with the embryonic development of the human aortic arch system, showing that six pairs of aortic arches connect the two primitive ventral and dorsal aortae (Fig. 82-1). Most portions of the first, second, and fifth arches regress. The third arches become the carotid arteries. A branch from the ventral bud of the sixth arch meets the lung bud to form the pulmonary artery. On the right side, the dorsal contribution to the sixth arch disappears; on the left, it persists as the ductus arteriosus.
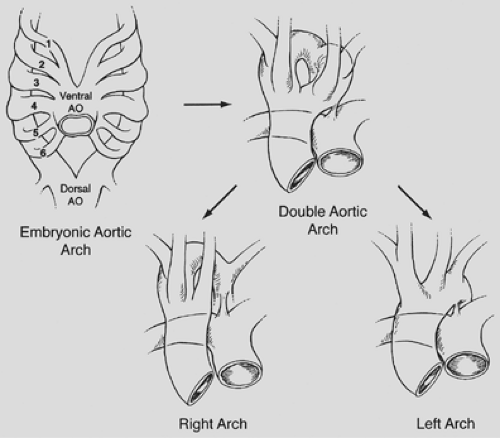
The formation of a vascular ring depends on preservation or deletion of specific segments of the embryonic aortic arch complex. To help visualize this, Edwards33 proposed a schematic model with a double aortic arch system and bilateral ductus arteriosus. By convention, the “location” of the aortic arch is determined by its relationship to the trachea. In the normal arch
formation, the right fourth arch regresses, leaving a left aortic arch system (i.e., apex of aortic arch to the left of the trachea). If both fourth arches persist, a double aortic arch is formed. If the left fourth arch regresses, a right aortic arch system is created (apex of aortic arch to the right of the trachea). This is schematically demonstrated in Figure 82-2.
formation, the right fourth arch regresses, leaving a left aortic arch system (i.e., apex of aortic arch to the left of the trachea). If both fourth arches persist, a double aortic arch is formed. If the left fourth arch regresses, a right aortic arch system is created (apex of aortic arch to the right of the trachea). This is schematically demonstrated in Figure 82-2.
Table 82-2 History of Vascular Ring Surgery | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Clinical Features, Diagnoses, and Surgical Treatment
Double Aortic Arch
A double aortic arch is the most common complete vascular ring that causes tracheoesophageal compression. Potts and associates68 noted that patients typically present in the first months of life with symptoms of stridor, respiratory distress, and a cough that sounds like a seal’s bark. A simple cold may precipitate severe respiratory difficulty. The ascending aorta divides into two arches that pass around the trachea and esophagus and join posteriorly to form the descending aorta (see Fig. 82-2). The authors8 showed that in two-thirds of these infants, the right-sided (posterior) arch is dominant, and in one-third, the left-sided (anterior) arch is dominant. Rarely, the arches are of equal size (balanced arches). The carotid and subclavian arteries originate symmetrically and separately from each arch. The tight, constricting ring thus formed compresses the trachea and esophagus.
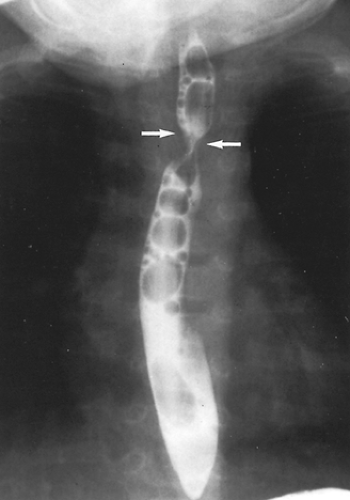
The diagnosis can be suspected on examination of the chest radiograph because the location of the aortic arch in relation to the trachea is indeterminate. In addition, the tracheal air
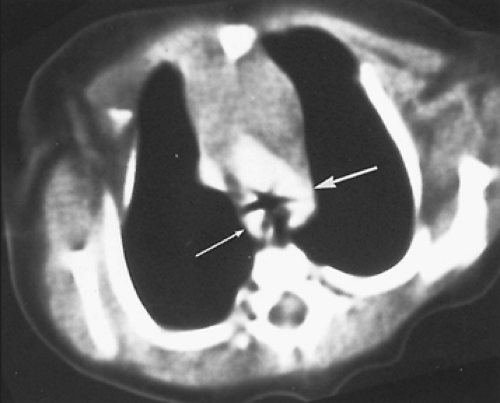
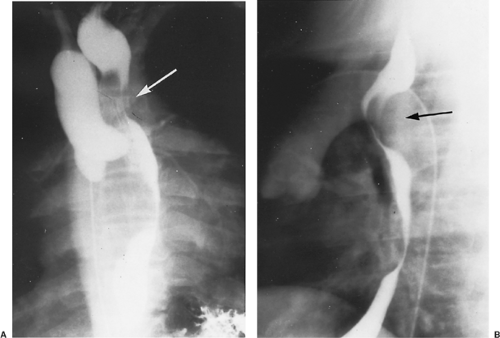
column often shows compression that is more prominent on the side of the dominant arch. Although, for many years, a barium esophagogram was the next diagnostic procedure of choice, currently we recommend a computed tomography (CT) scan with contrast.15 The double aortic arch on an anteroposterior esophagogram appears as bilateral indentations of unequal size that persist in location (Fig. 82-3). However, the barium esophagogram does not define the precise anatomy of the vascular ring. The authors’15 review and that of Lambert58 have shown that CT scanning is very accurate in the identification of vascular anomalies of the aortic arch and great vessels. A double aortic arch is diagnosed with certainty when both limbs are patent and enhanced with the administration of contrast material (Fig. 82-4).
column often shows compression that is more prominent on the side of the dominant arch. Although, for many years, a barium esophagogram was the next diagnostic procedure of choice, currently we recommend a computed tomography (CT) scan with contrast.15 The double aortic arch on an anteroposterior esophagogram appears as bilateral indentations of unequal size that persist in location (Fig. 82-3). However, the barium esophagogram does not define the precise anatomy of the vascular ring. The authors’15 review and that of Lambert58 have shown that CT scanning is very accurate in the identification of vascular anomalies of the aortic arch and great vessels. A double aortic arch is diagnosed with certainty when both limbs are patent and enhanced with the administration of contrast material (Fig. 82-4).
As described by Lowe and associates,57 one clue to an arch anomaly, specifically a double aortic arch or a right aortic arch with retroesophageal subclavian artery and a ligamentum arteriosum, is the “four artery sign.” This is seen on sections cephalad to the aortic arch and consists of two dorsal subclavian arteries and two ventral carotid arteries spaced evenly around the trachea. The sign is present when the two dorsal subclavian arteries arise directly from the aorta and not from a brachiocephalic artery (Fig. 82-5). Three dimensional (3D) reconstruction of the CT scan provides superb anatomic detail, allowing clear planning of the surgical approach and reducing the chance of error in selecting the site and approach for ring division (Fig. 82-6).
Magnetic resonance imaging (MRI), as described by Van Son and associates,75 also demonstrates the arch and vessel anatomy. MRI provides the same information as CT without ionizing radiation or the need for intravenously administered contrast material. However, if the child moves during the scan, there will be motion artifact, making the analysis difficult. Another disadvantage of MRI is the length of time required for the study, thus necessitating sedation. Currently we feel the ultrafast CT scan with contrast is the test of choice. Cardiac catheterization is recommended now only for infants with associated congenital cardiac anomalies.
Bronchoscopy is sometimes obtained as the initial study because of the presentation with stridor and shows typical external compression of the trachea. All vascular ring patients should have bronchoscopy as part of their evaluation, either prior to
surgical intervention or more commonly at the time of their operation. They should also all have an echocardiogram, as 12.4% of these patients have associated intracardiac lesions.
surgical intervention or more commonly at the time of their operation. They should also all have an echocardiogram, as 12.4% of these patients have associated intracardiac lesions.
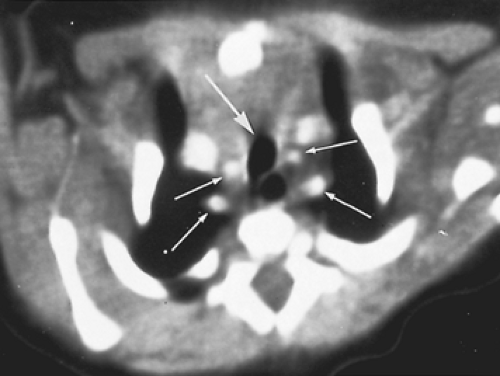 Figure 82-5. Same infant as in Fig. 82-4. The small arrows point to the four brachiocephalic vessels; the large arrow points to the trachea. |
All infants with double aortic arch should be operated on; a narrowed trachea, when further compromised by mucosal edema from even a mild upper respiratory infection, can cause hypoxic or apneic episodes. As Midulla and associates62 have reported, these children are also at risk for aortic dissection. In addition, as Heck,46 Othersen,64 and Angelini3 have reported, improper management of respiratory obstruction with prolonged intubation and nasogastric tube irrigation may lead to catastrophic erosion of an arch into the esophagus. We recently operated on a 4-month-old child hospitalized for 2 weeks with an endotracheal tube and a nasogastric tube. The esophagus had a 6-mm perforation caused by erosion of the left arch.
Surgical approach is determined by which arch is dominant. When the right arch is dominant (75%), the approach is through a left thoracotomy. When the left arch is dominant (18%), the approach is through a right thoracotomy. When the arches are balanced (7%), a left thoracotomy is preferred. If the patient has a significant intracardiac lesion, the ring can be divided through a median sternotomy. The left thoracotomy can be performed with a muscle-sparing technique, elevating the serratus anterior and the latissimus dorsi and entering the thorax through the fourth intercostal space. The vascular ring caused by the double aortic arch is released by dividing the lesser of the two arches, usually where it inserts into the descending aorta (Fig. 82-7). The definition of which arch is “smaller” is best made on the preoperative CT or MRI. In 30% to 40% of these patients, the site where the smaller arch inserts into the descending aorta
will be atretic. After applying the vascular clamps, the anesthesiologist carefully checks the carotid and radial pulses on both sides to ensure blood flow is not interrupted. The arch is then divided between the vascular clamps, and the stumps are oversewn with Prolene sutures; simple ligation should not be done. The ligamentum arteriosum is also divided. Careful dissection is then performed around the trachea and esophagus to lyse any residual adhesive bands. The recurrent laryngeal and phrenic nerves are identified and protected throughout the procedure. The mediastinal pleura is not sutured closed because this could contribute to scar formation, which might recreate the problems caused by the original vascular ring. For many years now, we have routinely not placed a chest tube but have simply used a plastic suction catheter to evacuate pleural air and have pulled this out as the skin is closed. One technical factor that the surgeon should be aware of is that when the clamps are placed on the vascular ring before ring division, the ring is temporarily tightened by inserting the clamps into the area between the ring and the trachea and esophagus. Patients will sometimes have a drop in their oxygen saturation and may require increased ventilatory pressures to provide adequate ventilation while the clamps are on the vascular ring.
will be atretic. After applying the vascular clamps, the anesthesiologist carefully checks the carotid and radial pulses on both sides to ensure blood flow is not interrupted. The arch is then divided between the vascular clamps, and the stumps are oversewn with Prolene sutures; simple ligation should not be done. The ligamentum arteriosum is also divided. Careful dissection is then performed around the trachea and esophagus to lyse any residual adhesive bands. The recurrent laryngeal and phrenic nerves are identified and protected throughout the procedure. The mediastinal pleura is not sutured closed because this could contribute to scar formation, which might recreate the problems caused by the original vascular ring. For many years now, we have routinely not placed a chest tube but have simply used a plastic suction catheter to evacuate pleural air and have pulled this out as the skin is closed. One technical factor that the surgeon should be aware of is that when the clamps are placed on the vascular ring before ring division, the ring is temporarily tightened by inserting the clamps into the area between the ring and the trachea and esophagus. Patients will sometimes have a drop in their oxygen saturation and may require increased ventilatory pressures to provide adequate ventilation while the clamps are on the vascular ring.
The postoperative care includes high humidity to loosen secretions; oxygen therapy when needed, as monitored by pulse oximetry; chest physiotherapy; and nasopharyngeal suctioning. Vigorous attempts should be made to achieve early extubation. Other helpful postoperative modalities include inhaled corticosteroids, albuterol treatments, and heliox. Results of surgical intervention are excellent, and no surgical mortality has resulted at Children’s Memorial Hospital from a double aortic arch procedure since 1952. Most patients are discharged within 48 hours of the operation. As Nikaidoh and associates63 have reported,
some children have residual noisy breathing for 6 months to 2 years, but in nearly all instances this gradually resolves.
some children have residual noisy breathing for 6 months to 2 years, but in nearly all instances this gradually resolves.
Right Aortic Arch
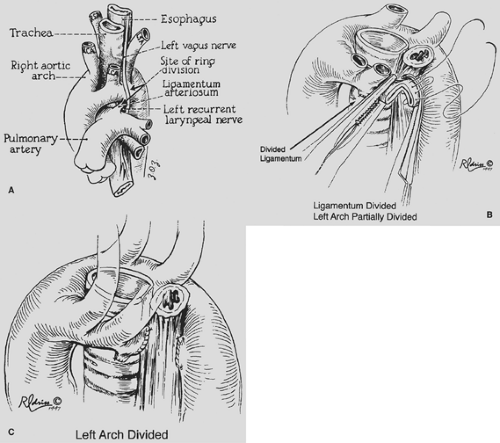
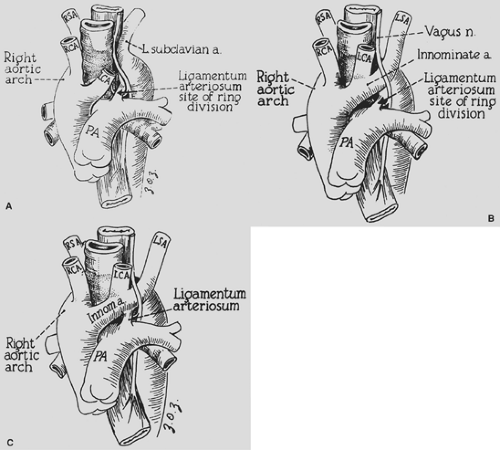
A right aortic arch with a left ligamentum arteriosum completing the vascular ring is almost as common as a double aortic arch. The ring, however, is usually not as tight, and children typically present somewhat later in life (6 to 12 months of age). Symptoms of stridor, barky cough, and respiratory distress are similar to those in infants with double aortic arch. In older children, dysphagia may be present. Some of these children eat very slowly and tend to be the last to leave the table because they have to chew their food carefully, as a learned procedure, to prevent choking. Embryologically, depending on the exact site or sites of interruption of the left fourth arch and the branching pattern to the left subclavian artery, left carotid artery, and ductus arteriosus, different configurations of right aortic arch are possible. Felson and Palayew35 showed that the two common variations are retroesophageal left subclavian artery (65%) and mirror-image branching (35%) (Fig. 82-8A,B). In either case it is the left ligamentum arteriosum between the descending aorta and the left pulmonary artery that completes the ring. Although D’Cruz and associates30 have reported that one-third of patients with tetralogy of Fallot and truncus arteriosus have a right aortic arch, our group did not find a reverse association with a specific cardiac defect when a vascular ring is formed by a right arch. In most infants with a cardiac defect and a right aortic arch, there is mirror-image branching with the ligamentum from the innominate artery to the pulmonary artery, so a vascular ring is not formed (Fig. 82-8C).
The diagnosis is suggested by the chest radiograph, which shows an aortic arch to the right of the tracheal air column. In children who have a ligamentum completing the ring, compression of the trachea by the aorta is often quite impressive. Barium esophagogram used to be the diagnostic procedure
of choice (Fig. 82-9). However, using the same analysis as for a double aortic arch, we feel that CT imaging is now the best diagnostic procedure. This reveals the precise anatomy of the ring, including the identification of a Kommerell’s diverticulum as part of the pathology. Chun and associates26 have emphasized the importance of an associated Kommerell’s54 diverticulum. This diverticulum is embryologically a remnant of the left fourth aortic arch that did not undergo complete involution. The diverticulum may independently compress the esophagus or trachea. Fisher and colleagues36 have noted the possible complications of ruptured aneurysm and aortic dissection. This aneurysmal dilatation should be resected if present and usually necessitates transfer of the left subclavian artery to the left carotid artery. An example of an MRI in a patient with a Kommerell’s diverticulum, right aortic arch, left ligamentum, and retroesophageal left subclavian artery is shown in Figure 82-10.
of choice (Fig. 82-9). However, using the same analysis as for a double aortic arch, we feel that CT imaging is now the best diagnostic procedure. This reveals the precise anatomy of the ring, including the identification of a Kommerell’s diverticulum as part of the pathology. Chun and associates26 have emphasized the importance of an associated Kommerell’s54 diverticulum. This diverticulum is embryologically a remnant of the left fourth aortic arch that did not undergo complete involution. The diverticulum may independently compress the esophagus or trachea. Fisher and colleagues36 have noted the possible complications of ruptured aneurysm and aortic dissection. This aneurysmal dilatation should be resected if present and usually necessitates transfer of the left subclavian artery to the left carotid artery. An example of an MRI in a patient with a Kommerell’s diverticulum, right aortic arch, left ligamentum, and retroesophageal left subclavian artery is shown in Figure 82-10.
The surgical approach is through a left thoracotomy. After careful dissection and identification of the configuration of the aortic arch, the ligamentum arteriosum is identified as compressing the esophagus. The ring is released by dividing the ligamentum between vascular clamps and oversewing the stumps with Prolene suture. Any adhesive bands around the esophagus are lysed.
In the recent experience at our institution that was published by the author and colleagues,14 10 children required additional surgery because their vascular ring (ligamentum) was divided without addressing the associated Kommerell’s diverticulum. All were symptomatic, with recurrent respiratory symptoms or recurrent dysphagia. All patients responded to reoperation with resection of the diverticulum and transfer of the left subclavian artery to the left carotid artery with resolution of airway symptoms (Figs. 82-11, 82-12 and 82-13). Because of the success of these reoperations, the primary strategy for patients with a right aortic arch, left ligamentum, and Kommerell’s diverticulum should be division of the ligamentum, resection of Kommerell’s diverticulum, and left subclavian artery transfer. We have done this as a primary procedure in eight patients.
A very rare group of children with a right aortic arch and left ligamentum have a left-sided descending thoracic aorta, the so-called circumflex aorta. Robotin and associates70 have described an aortic “uncrossing” operation for these rare patients (3 of 468 patients in their series of patients with vascular rings). This procedure is performed through a median sternotomy
with cardiopulmonary bypass and hypothermic circulatory arrest. The aortic arch is mobilized, divided, and brought in front of the tracheobronchial tree, then reanastomosed end-to-side to the lateral aspect of the ascending aorta (Fig. 82-14). All three patients described by Robotin had prior ligamentum division via left thoracotomy. We have done an aortic “uncrossing” in two patients.
with cardiopulmonary bypass and hypothermic circulatory arrest. The aortic arch is mobilized, divided, and brought in front of the tracheobronchial tree, then reanastomosed end-to-side to the lateral aspect of the ascending aorta (Fig. 82-14). All three patients described by Robotin had prior ligamentum division via left thoracotomy. We have done an aortic “uncrossing” in two patients.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree