Understanding and addressing complications of percutaneous coronary intervention (PCI) is critical to the practice of interventional cardiology.
It is critical to appreciate and explain the possible complications of PCI in order to provide proper informed consent to the patient. It is also critical to be vigilant and to recognize potential complications at an early stage in order to try to reverse the adverse outcome, as the most common cause of all post-PCI deaths is from a procedural complication rather than from a preexisting cardiac condition (
1).
Some of the complications are generic to all coronary angiography procedures, while others are specific to coronary intervention. Events such as death, myocardial infarction (MI), and bleeding occur at higher rates for interventional procedures as there is direct manipulation of the coronary arteries often accompanied by prolonged procedural time, complexity, and the use of higher-intensity anticoagulation (
Tables 27-1 and
27-2). Complications of PCI can occur at any step of the procedure from the administration of sedation to the transfer as the patient leaves the laboratory. The goal of this chapter is to incorporate the latest statistics and guidelines regarding the diagnosis and management of complications of PCI.
MORTALITY
Mortality is the most serious complication of PCI. The cause can be secondary to any of the other complications listed in this chapter. Inhospital mortality is very rare with diagnostic angiography (<0.1%), but the rate increases over 12-fold with the addition of coronary intervention to 1.27%. The mortality rate greatly varies, depending on the urgency of PCI, with a range of 0.65% in elective PCI to 4.81% in ST-segment elevation myocardial infarction (STEMI) (
2).
The strongest risk predictors of in-hospital mortality used in the National Cardiovascular Data Registry (NCDR) Cath-PCI risk model include advanced age, cardiogenic shock, history of congestive heart failure, peripheral vascular disease, chronic lung disease, worsening renal function, New York Heart Association functional Class IV patients, urgency of PCI status, and whether or not the patient is having a STEMI (
2).
COMPLICATIONS OF VASCULAR ACCESS
The first part of any procedure begins with vascular access. The major complications are femoral artery pseudoaneurysm, arteriovenous fistula, and bleeding (including retroperitoneal hemorrhage). As seen in
Table 27-1, the incidence of these complications is increased in procedures in which PCI is performed compared with that in a strictly diagnostic procedure (
3). Specific discussion of each of these complications is beyond the scope of this chapter.
COMPLICATIONS OF ATHEROEMBOLISM (STROKE, PERIPROCEDURAL MI, CHOLESTEROL EMBOLIZATION)
Advancing large bore guiding catheters, or even 6-Fr catheters across a diseased aorta (either abdominal or thoracic) heavily burdened with atherosclerotic plaques may result in thromboembolic
events, resulting in peripheral ischemia, renal failure, or stroke. Peripheral atheroembolism with obstruction of small arteries and arterioles by cholesterol crystals is known as the cholesterol embolization syndrome (CES). This is relatively rare (incidence of 0.75%—1.4%). Typically, this is diagnosed by one of the three typical cutaneous signs: livedo reticularis, blue toe syndrome/trash foot, or frank digital gangrene,in addition to laboratory evidence of an elevated eosinophil count. In-hospital mortality is as high as 16% in those patients with definite CES as multiorgan embolization can often lead to multiorgan failure (
4).
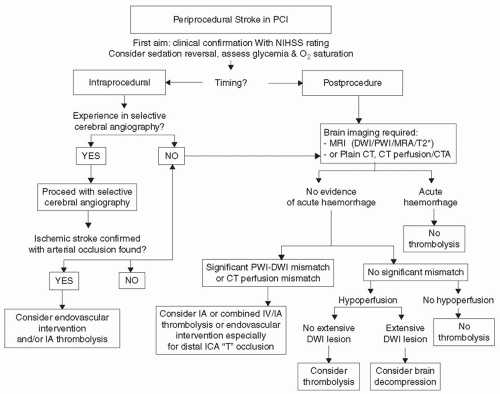
Atheroembolism can also obstruct the arteries of the brain, causing a cerebral vascular accident (CVA) or transient ischemic attack (TIA). The overall incidence of TIA or CVA is quite low after PCI (
5). There are various multivariate predictors of in-hospital CVA (
Table 27-3). The most common symptoms of a perioperative TIA or CVA are motor or speech deficits. In-hospital death can occur in up to 25% of those with a CVA, but increased mortality is not expected with a TIA (
5). If the stroke occurs during the procedure, then consideration should be given for an emergent neurointervention with resultant cerebral angiography and intervention if an ischemic stroke with arterial occlusion is found. If the stroke occurs after the procedure and gets confirmed by advanced imaging, then thrombolysis can be considered after hemorrhagic stroke is ruled out (
Fig. 27-1) (
6).
Intracoronary atheroembolism is one mechanism of periprocedural MI. Periprocedural MI is considered a major adverse cardiac event and a core measure in the recent SCAI quality assessment and improvement position statement (
7). A meta-analysis of 15 observational studies found that periprocedural MIs were linked with worse in-hospital and long-term outcomes (
8). According to the new universal definitions of MI, a PCI-related MI is the increase of biomarkers greater than three times the 99th percentile
of the upper reference limit (
9). While it is common (24%) to have some evidence of myonecrosis (any enzyme level above the upper limits of normal) after a percutaneous intervention, it is rarer (8%) to have a true periprocedural MI (
10). Besides intracoronary atheroembolism, other causes of periprocedural MI include occluded side branches, no-reflow, vessel perforation, vasospasm, acute stent thrombosis, and dissection. The management of the periprocedural MI depends on its underlying cause.
ARTERIAL DISSECTION
The guide catheter itself can cause coronary dissection with or without extension to the aortic root. More commonly, coronary dissection is caused by advancement of the coronary guidewire or by balloon inflation. Large visible dissections have been described in up to 30% of all angioplasty procedures (
11). Previously, this was a significant risk factor for acute/abrupt vessel closure, which occurs rarely in the era of coronary stenting. The National Heart Lung and Blood Institute (NHLBI) classifications of coronary dissections are seen in
Table 27-4 (
12). Types E and F may represent the additional complication of intracoronary thrombus.
Catheter-related dissection is a much rarer event, with a reported incidence of 0.06% (
13). The mechanism of the dissection is likely because of mechanical trauma to the intima of the vessel (either normal or with plaque) from a catheter that is wedged into the wall rather than lying coaxial. A jet of contrast from an abnormally seated catheter might also cause or worsen a coronary dissection. Risk factors for catheter-induced coronary artery dissection include left main disease, use of Amplatz-shaped catheters, acute MI, extensive catheter manipulation, vigorous contrast injection, deep intubation of the catheter within the coronary artery (sometimes caused by deep inspiration by the patient), and variant anatomy of the coronary ostia (
14). Stenting the dissected area remains the standard of treatment. If a guide catheter-induced dissection is noticed, this should be fixed before the initial intended lesion that prompted the PCI. The rationale is that if the dissection is not fixed, it can propagate forward and cause abrupt vessel closure or propagate backward and cause aortic dissection.
The incidence of aortic dissection caused by catheter trauma is very rare, 0.02%.
Table 27-5 shows a classification scheme for extension of an aortic dissection (
15). Almost all cases of retrograde extension of dissection are from the right coronary artery (RCA). Class I and II lesions have a good prognosis, and just require stenting of the coronary dissection with close clinical follow-up. It is reasonable to follow the evolution of the dissection with imaging modalities (CT or TEE). If the patient remains stable over the next 24 to 48 hours of hospitalization, then he or she can be safely discharged without the expectation for further complication (
15). To reduce the chance of extension, the systolic blood pressure must be optimally controlled. However, antiplatelet therapy should not be suspended with a freshly placed coronary stent. Class III aortic dissections generally should be treated surgically and are associated with a much higher mortality rate. If surgery is not a possibility, then the entrance of the dissection in the coronary should be stented to avoid further propagation of the aortic dissection.