Fig. 16.1
ECG and His bundle electrogram of Complete Heart Block. Leads I, V1, V6 with an atrial (A) electrogram, a His bundle electrogram (H), and ventricular (V) electrogram demonstrating CHB with a His bundle (H) escape rhythm
The anatomy and electrophysiology of the AV node has been described in Chap. 15.
While there are various classifications regarding the age and mechanism of complete heart block (CHB) in a young person, for the purpose of this discussion, congenital CHB includes both immune-mediated and nonimmune-mediated heart block that is diagnosed in utero, at birth, or in the neonatal period (0–27 days after birth). Isolated congenital CHB occurs without coexisting structural heart disease. Acquired CHB can be a complication of surgical repair, infection, neoplasm, or other rare occurrences; it can occur later in childhood or adolescence spontaneously without identifiable cause, and persist or resolve (Table 16.1).
Table 16.1
Etiology of complete heart block
Isolated complete heart block—No associated structural heart disease |
• Congenital HB—Immune mediated |
• Congenital HB—nonimmune mediated |
• Cardiac manifestation of neuromuscular disease |
– Emery–Dreifuss muscular dystrophy |
– Kearns–Sayre syndrome |
– Myotonic dystrophy |
• Genetic associations |
– Idiopathic AV conduction disease |
– PRKAG2—ventricular preexcitation and AV block |
– SCN5A |
– NKX2.5 |
• Fibrosis and sclerosis |
– Fibrosis and sclerosis of the conduction system accounts for about one-half of cases of AV block in adults |
– Lenegre’s disease is a progressive, fibrotic, sclerodegenerative disease of the conduction system in younger individuals associated with slow progression to CHB and may be hereditary |
• Valvular disease: Calcification and fibrosis of the aortic or mitral valve rings can extend into the conducting system |
• Cardiomyopathies |
– Hypertrophic obstructive cardiomyopathy |
– Infiltrative processes such as amyloidosis and sarcoidosis |
• Hyperthyroidism, myxedema, and thyrotoxic periodic paralysis |
• Malignancies: Such as Hodgkin’s disease and other lymphomas; multiple myeloma; and cardiac tumors |
• Drugs: A variety of drugs can impair conduction and cause AV block |
• Ischemic heart disease |
Complete heart block associated with congenital heart disease, most commonly: |
• L-transposition of the great arteries |
• Single ventricle |
• Heterotaxy syndrome |
Congenital CHB was first described in 1901 by Morquio, who also noted a familial occurrence and an association with Stokes–Adams attacks and death. The presence of fetal bradycardia (40–80 bpm) as a manifestation of CHB was first noted in 1921. The incidence of congenital CHB in the general population varies between 1 in 15,000 and 1 in 22,000 live-born infants, the majority of which are autoimmune mediated.
Autoimmune-Mediated CHB
Neonatal Lupus
Complete heart block, hepatobiliary disease, malar rash, thrombocytopenia and, less frequently, myocarditis comprise neonatal lupus primarily presenting in utero or in the neonate. Frequently, the only manifestation of neonatal lupus, and by extension an autoimmune abnormality in the mother, is CHB in the newborn. Since skin, liver, and blood cells regenerate, the effect of passively acquired antibodies in these organs in the fetus/newborn resolves, usually in the first 6 months of life. However, cardiac cells are not regenerative and thus CHB from neonatal lupus is permanent.
In the absence of congenital heart disease, neonatal lupus is responsible for 60–90 % of cases of congenital CHB. Maternal IgG auto-antibodies to SSA/Ro and/or SSB/La from a mother with an autoimmune connective tissue disorder, most frequently lupus erythematosus, cross the placenta to the fetus during the first trimester. Anti-Ro/SSA and/or anti-La/SSB antibodies bind to fetal cardiac tissue, leading to immune-mediated injury to the AV node and its surrounding tissue.
Both Ro/SSA and La/SSB antigens are abundant in fetal heart tissue between 18 and 24 weeks. Apoptosis induces translocation of Ro/SSA and La/SSB to the surface of fetal cardiomyocytes; the maternal, transplacental anti-Ro and anti-La antibodies then bind to the surface of the fetal cardiomyocytes and induce the release of tumor necrosis factor by macrophages, resulting in fibrosis. In addition to inducing tissue damage, anti-Ro/SSA and/or anti-La/SSB antibodies inhibit calcium channel activation of the cardiac L- and T-type calcium channels themselves; L-type channels allow action potential propagation and conduction in the AV node. The sinoatrial (SA) node also may be involved; sinus bradycardia has been described in 3.8 % percent of fetuses but is usually not permanent.
Immune-mediated CHB accounts for almost all cases of CHB presenting in utero or during the neonatal period. Rarely, it may explain a few cases occurring later (approximately 5 %). Approximately 2 % of babies born to SSA/SSB + mothers, the majority of whom are often asymptomatic, have CHB; however, the recurrence rate for future offspring may be as high as 17 %.
Clinical Manifestations of Neonatal Lupus
The patients with the neonatal lupus syndrome tend to present earlier than those with CHB not due to neonatal lupus.
Congenital CHB may present with fetal bradycardia between 18 and 28 weeks of gestation. In utero detection is made by echocardiography, which can estimate the fetal PR interval. Complications of CHB in utero include hydrops fetalis, myocarditis, endocardial fibroelastosis, pericardial effusion, and spontaneous intrauterine fetal death. Advanced AV block and cardiomyopathy can occur within one week of normal fetal echo without first-degree AVB (Fig. 16.2).
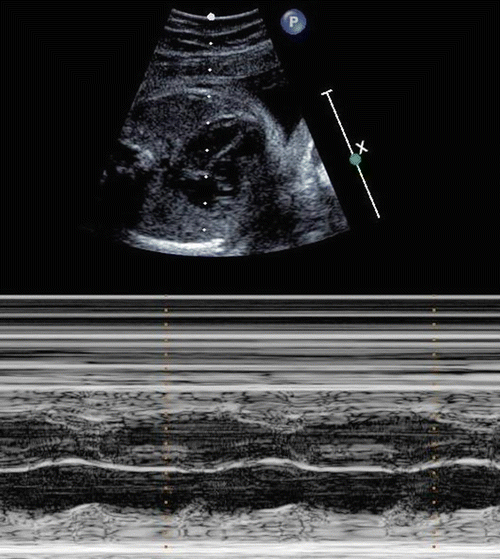

Fig. 16.2
Fetal echocardiographic diagnosis of complete AV block. M mode fetal echo demonstrating atrial (A) and ventricular contractions (V) and complete heart block
Infants who present with heart block in utero, but who survive until birth, have a high neonatal mortality rate. In one report, 6 of 22 such infants (27 %) died within one week of birth. In another series, 10 of 107 (9 %) died within the first 3 months. Infants born before 34 weeks have a higher mortality rate than those born later (52 % vs. 9 %). Infants with first- or second-degree heart block at birth can progress to CHB.
As in the fetus, the cardinal finding of CHB in the neonate is a slow heart rate. In addition to bradycardia, other clinical clues in the neonate include intermittent cannon waves in the neck, a first heart sound that varies in intensity, and intermittent gallops and murmurs. As with cases presenting in utero, almost all presenting in the neonatal period are immune mediated. The newborn at greatest risk has a rapid atrial rate, often 150 bpm or faster, and a ventricular rate less than 50 bpm.
Similar to acquired CHB, the ECG most commonly shows a narrow QRS complex due to a junctional escape rhythm. First- or second-degree heart block found in infants at birth can progress to CHB. The outcome for patients diagnosed as neonates is better than for those diagnosed in utero.
Outcome
In the immediate postnatal period, CHB with low escape rhythm may be supported with isoproterenol or postnatal pacemaker insertion. Overall mortality rate is 4–29 %; mortality rate before age 3 months is 15 %, and the mortality rate from cardiac complications at any age is 14 %.
Data regarding prevention of immune-mediated CHB in at-risk fetuses is not conclusive. However, recent data suggest that prophylactic use of hydroxychloroquine and/or dexamethasone therapy in mothers with known anti-Ro/SSA and anti-La/SSB autoimmune disease has been associated with a decreased incidence of fetal heart block. Dexamethasone has been demonstrated to reverse PR prolongation when started during fetal life.
Complete Heart Block, Nonimmune Mediated
Nonimmune causes of congenital CHB include various structural cardiac defects, particularly congenitally corrected transposition of the great arteries, AV discordance, or polysplenia with AV canal defect. Additionally, several genetic disorders such as familial atrial septal defect and Kearns–Sayre syndrome have been identified. In most cases, CHB is characterized pathologically by fibrous tissue that either replaces the AV node and its surrounding tissue or by an interruption between the atrial myocardium and the AV node.
Presentation in Childhood
As many as 40 % of cases of CHB do not present until childhood (mean age 5 to 6 years) (Fig. 16.3). Few of these patients have neonatal lupus. The diagnosis is usually made when detecting a slow pulse or by presentation with fatigue or syncope, though syncope can herald a more ominous course.
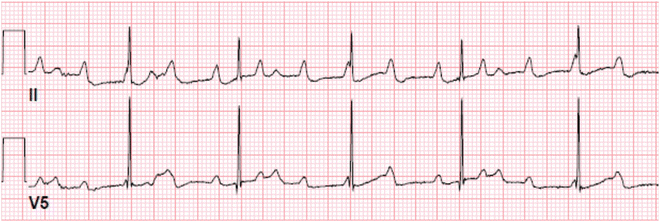

Fig. 16.3
Complete heart block with junctional escape. A 2-year-old presenting with asymptomatic bradycardia. ECG demonstrates complete heart block with junctional escape
Complete heart block may be intermittent when first detected, but usually becomes persistent in later childhood. It was once presumed that most unexplained CHB diagnosed for the first time beyond infancy was congenital in origin and has escaped notice because of a higher ventricular rate and the absence of symptoms. However, given that prenatal ultrasound is now well developed and in wide use, it appears that cases not detected in fetal life, in fact have preserved AV conduction at birth and acquire progressive AV nodal disease thereafter. Findings from different centers are consistent with the notion of progressive AV nodal disease. In one report, the number of childhood case referrals remained constant from 1980 to 1998 despite the introduction of fetal echocardiography and the wide availability of heart rate monitoring during pregnancy and labor. Second, in a series of 102 patients who were asymptomatic through age 15 and were followed for 7–30 years thereafter, a slow decline in ventricular rate was noted with increasing age. Some patients present with bradycardia-related symptoms, including reduced exercise tolerance, presyncope, or syncope. Sudden death has also been described.
AV Conduction Disease, Genetic Association
AV conduction abnormalities can be the major cardiac manifestation of neuromuscular diseases or of other genetic mutations. Emery–Dreifuss muscular dystrophy occurs as an X-linked trait or an autosomal dominant mutation; X-linked Emery–Dreifuss occurs due to emerin mutations and an autosomal dominant inheritance pattern is due to lamin A/C mutations. Conduction system abnormalities in Emery–Dreifuss muscular dystrophy are progressive and can occur throughout the conduction system. Kearns–Sayre syndrome is a mitochondrial disorder with conduction abnormalities. Myotonic dystrophy is an autosomal dominant disorder with progressive conduction abnormalities. Mutations in NKX2.5 can be associated with AV conduction disease, as well as congenital heart defects; AV block is progressive, with CHB developing usually by the third decade of life.
Acquired CHB
Acquired CHB in children most commonly occurs due to surgical repair of congenital heart disease. Infrequently, AV block can occur due to other causes, such as catheter-based interventions or infectious myocarditis. Other rare causes of AV block are listed in Table 16.1; many of these occur more commonly in adult patients and only rarely occur in children.
Surgical CHB
The incidence of early postoperative heart block associated with surgical correction or palliation of congenital heart disease is known to be between 0.7 and 3 %, depending on the cardiac lesion. However, certain surgeries or congenital defects carry a higher risk of surgical heart block. CHB after surgical repair of congenital heart disease is most associated with VSD repair. Weight under 4 kg and inlet VSD have been identified as risk factors for permanent surgical heart block requiring a pacemaker.
For some patients, early surgical complete heart block (SCHB) is temporary and AV conduction returns. Surgical AV block following surgery for congenital heart disease resolves in 2/3 of patients by the ninth postoperative day. Surgical AV block is considered permanent when persisting >7–14 days. AV conduction that recovers late (>14days) after surgery is known to occur in 12 % and has been reported to occur anywhere between 3 weeks and 7 years postoperatively. CHB can also occur late after surgical correction, including those patients with transient AV block in the immediate postoperative period. The incidence of late postoperative heart block has not been well established. Recently, it has been reported to be between 0.3 and 0.7 %. The development of late postoperative AV block has also been hypothesized to have a genetic component. Late recurrence of transient SCHB does not appear to be related to the electrophysiologic properties of the conduction system as determined by electrophysiologic studies performed following recovery of the transient SCHB.
CHB Due to Catheter-Based Intervention
Diagnostic catheter-based procedures have an extremely low incidence of CHB. Interventional catheter-based procedures have a variable risk of CHB. Temporary heart block is reported to occur in 0.4 % of interventional procedures for ASD/PFO closure. CHB requiring pacemaker implantation as a result of ASD/PFO closure is an extremely rare occurrence, reported in 1 patient of >28,000 in a meta-analysis. However, conduction abnormalities requiring pacemaker implantation are more common after transcatheter aortic valve implantation (TAVI), reported in 6–42 % of cases. CHB during interventional electrophysiology studies with ablation has been reported to be as high as 0.9 %; however, this report includes temporary AV block. Complete AV block associated with ablation procedures requiring a pacemaker is rare.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


