Treatment of patients with in-stent restenosis (ISR) remains a challenge. This study sought to compare the efficacy of everolimus-eluting stents (EESs) and drug-eluting balloons (DEBs) with paclitaxel in patients with ISR. A pooled analysis of the Restenosis Intra-Stent of Drug-Eluting Stents: Drug-Eluting Balloon vs Everolimus-Eluting Stent (RIBS IV) and Restenosis Intra-Stent of Bare-Metal Stents: Drug-Eluting Balloon vs Everolimus-Eluting Stent (RIBS V) randomized trials was performed using patient-level data. In both trials, EESs were compared with DEBs in patients with ISR (RIBS V included 189 patients with bare-metal ISR; RIBS IV included 309 patients with drug-eluting ISR). Inclusion and exclusion criteria were identical in both trials. A total of 249 patients were allocated to EES and 249 to DEB. Clinical follow-up at 1 year was obtained in all (100%) patients and late angiography (median 249 days) in 91% of eligible patients. Compared with patients treated with DEBs, patients treated with EESs obtained better short-term results (postprocedural minimal lumen diameter 2.28 ± 0.5 vs 2.12 ± 0.4 mm, p <0.0001). At follow-up, patients treated with EESs had larger in-segment minimal lumen diameter (primary end point 2.16 ± 0.7 vs 1.88 ± 0.6 mm, p <0.0001; absolute mean difference 0.28 mm; 95% confidence interval [CI] 0.16 to 0.40) and net lumen gain (1.33 ± 0.6 vs 1.00 ± 0.7 mm, p <0.0001) and had lower %diameter stenosis (19 ± 21% vs 28 ± 22%, p <0.0001) and binary restenosis rate (8.7% vs 15.7%, p = 0.02). Consistent results were observed in the in-lesion analysis. No interactions were found between the underlying stent type and treatment effects. At 1-year clinical follow-up, the composite of cardiac death, myocardial infarction, and target vessel revascularization was significantly reduced in the EES arm (8.8% vs 14.5%, p = 0.03; hazard ratio 0.59, 95% CI 0.31 to 0.94) mainly driven by a lower need for target vessel revascularization (6% vs 12.4%, p = 0.01, hazard ratio 0.46, 95% CI 0.25 to 0.86). This pooled analysis of the RIBS IV and RIBS V randomized trials demonstrates the superiority of EES over DEB in the treatment of patients with ISR.
Treatment of patients with in-stent restenosis (ISR) remains a challenge. In this setting, both drug-eluting stents (DESs) and drug-eluting balloons (DEBs) with paclitaxel are currently recommended. To better ascertain the relative efficacy of everolimus-eluting stents (EESs) versus DEBs in patients with ISR, we performed a pooled analysis of the Restenosis Intra-Stent of Drug-Eluting Stents: Drug-Eluting Balloon vs Everolimus-Eluting Stent (RIBS IV) (in patients with DES-ISR) and Restenosis Intra-Stent of Bare-Metal Stents: Drug-Eluting Balloon vs Everolimus-Eluting Stent (RIBS V) (in patients with bare-metal stent [BMS]-ISR) randomized trials, using patient-level data. In addition, we carefully evaluated potential interactions according to the underlying substrate (BMS-ISR vs DES-ISR) for most relevant angiographic and clinical outcome measures.
Methods
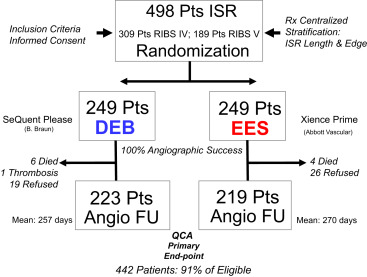
The RIBS V study was a prospective multicenter, open-label, controlled, randomized clinical trial that compared the results of DEBs versus those of EESs in patients with BMS-ISR ( ClinicalTrials.gov Identifier: NCT01239953 ) ( Online Appendix ). The RIBS IV study was a prospective multicenter, open-label, controlled, randomized clinical trial that compared the results of DEBs versus those of EESs in patients with DES-ISR ( ClinicalTrials.gov Identifier: NCT01239940 ) ( Online Appendix ). Inclusion and exclusion criteria were identical in the 2 trials and largely similar to those used in previous RIBS trials. These studies started simultaneously at 25 Spanish sites, although RIBS IV required a longer time to complete enrollment. From January 2010 to January 2012, 189 patients with BMS-ISR were randomly allocated to DEB (n = 95) or EES (n = 94) in RIBS V. From January 2010 to August 2013, 309 patients with DES-ISR were randomly assigned to DEB (n = 154) or EES (n = 155) in RIBS IV ( Figure 1 ). Patients with ISR (>50% diameter stenosis on visual assessment) and angina or ischemia demonstration were eligible. Any type of BMS showing ISR was eligible in RIBS V, whereas any type of DES with ISR was eligible in RIBS IV. In both studies, patients with small vessels (≤2.0 mm in diameter), long lesions (>30 mm in length), or total occlusions (Thrombolysis In Myocardial Infarction = 0) were ineligible. Likewise, patients with very early (<1 month) ISR and those presenting with an acute myocardial infarction or with a large angiographic thrombus were excluded. However, patients with repeated interventions for ISR (including those with >1 stent layer) and those with edge-ISR were eligible. Patients with contraindications to aspirin or clopidogrel, those with a life expectancy <1 year, and those with anticipated difficulties for a late angiographic follow-up were not included. Written informed consent was obtained from all patients. Telephonic randomization was performed using a computer-generated code. In both studies, randomization (1:1) was stratified according to ISR length (≤ or >10 mm) and lesion location (intrastent vs edge-ISR). Data monitoring and analysis was organized by the coordinating center (Hospital Universitario Clínico, San Carlos, Madrid). These studies were separate investigators’ driven initiatives organized under the auspices of the Working Group on Interventional Cardiology of the Spanish Society of Cardiology with the support of unrestricted research grants from B. Braun Surgical and Abbott Vascular. The Fundación Interhospitalaria de Investigación Cardiovascular was the promoter. The studies were approved by the Institutional Ethics Committee of the participating centers. The primary end point in the 2 studies was the comparison of the in-segment minimal lumen diameter at late follow-up between EES and DEB.
The interventional protocol was identical in the 2 trials. In brief, all patients were pretreated with aspirin and clopidogrel. Unfractionated heparin was used during the procedure targeting for an activated clotting time >250 seconds. The protocols emphasized the importance of ensuring an adequate lesion predilation with high pressures but, at the same time, of avoiding damaging the adjacent coronary segments. Special care was taken to tackle underexpanded stents using noncompliant balloons at very high pressures. Once adequate lesion predilation was achieved, patients received the allocated intervention covering the entire treated segment to prevent “geographical miss” phenomena. Patients allocated to DEB were treated using a specific paclitaxel-DEB (SeQuent Please; B. Braun Surgical, Melsungen, Germany) using 1.1/1 balloon-to-artery ratio and nominal pressures (12 to 14 atm) for 60 seconds. Crossover to bailout stenting was discouraged and only permitted in cases with dilation failure or major dissections. Patients allocated to EES (Xience Prime; Abbott Vascular, Abbott Park, Illinois) were treated selecting a 1.1/1 balloon-to-artery ratio and high (>14 bar) pressures. In this arm, final postdilation with noncompliant balloons was recommended but left to the operator’s discretion. Guidance by intracoronary imaging was suggested in the 2 trials, but the use of this strategy was also left to the operator’s criteria. Serial assessment of cardiac biomarkers and 12-lead electrocardiograms were systematically obtained. All the patients were to take aspirin indefinitely and clopidogrel for 1 year after EES implantation and for 3 months after DEB.
In both studies, late angiographic follow-up was scheduled at 6 to 9 months, although early angiography was recommended in patients with symptoms. The electronic case report forms were identical. These were completed at the sites and submitted to the coordinating center. A dedicated database designed for the RIBS studies was used. In patients with adverse events, all source documents were requested. A central clinical events committee, whose members were unaware of the treatment assignments, adjudicated all adverse events. Deaths were considered cardiac unless a noncardiac cause could be demonstrated. The diagnosis of myocardial infarction has remained unchanged in the RIBS trials and included (1) prolonged (>30 minutes) chest pain, (2) increase in creatine-kinase levels more than twice the local upper normal values (with abnormal MB fraction), and (3) development of new persisting ischemic electrocardiographic changes (with or without new pathologic Q waves). By protocol, all repeated interventions had to be clinically justified. Angiograms of all patients requiring target vessel revascularization were carefully reviewed at the core laboratory to identify the exact site of the reintervention. Stent thrombosis was analyzed using the Academic Research Consortium definition.
Angiograms were analyzed at the same central core laboratory by trained and blinded personnel using a standard methodology. Lesion morphology was assessed using the Mehran and the American College of Cardiology/American Heart Association classifications. Three orthogonal projections were selected after the administration of intracoronary nitroglycerin avoiding vessel foreshortening and side-branch overlap. These projections were repeated after interventions and at late follow-up. A validated automatic edge-detection system (CAAS II System; Pie Medical, Maastricht, The Netherlands) was used for quantitative analysis. Both in-lesion and in-segment (lesion + treated segment + 5-mm margins) analyses were performed.
Categorical data were compared with the chi-square test or Fisher’s exact test as required. Data distribution normality was evaluated with the Kolmogorov–Smirnov test. Continuous data presented as mean (±SD) or median (interquartile range) were compared using the Student t test or the Mann–Whitney test. Main effect estimates are presented as relative risk or hazard ratio with their 95% confidence interval (CI). Event estimates are expressed as Kaplan–Meier estimates of the cumulative incidence at 1 year and were compared with the log-rank and Breslow’s exact tests. Hazard ratios (95% CI) were generated using Cox proportional hazard models and compared with the Wald test. Results of the main outcome measure were examined according to 10 prespecified clinical and angiographic variables. Analyses were performed according to the intention-to-treat principle, unless otherwise specified. The number needed to treat to prevent angiographic restenosis and need for target lesion revascularization was calculated. Interactions between the underlying stent type (namely BMS-ISR vs DES-ISR) were formally evaluated (2-way analysis of variance). Potential influence on treatment effects were assessed for most relevant outcome measures (including minimal lumen diameter, late loss, %diameter stenosis, target lesion revascularization, and the combined clinical outcome measure). In addition, to further adjust for potential imbalances in baseline characteristics among examined subgroups, a logistic regression model was generated to calculate the probability of receiving each treatment modality in each individual trial. Subsequently, sensitivity analyses were developed using a meta-analytic methodology (Stata 12.0). Finally, treatment effects were analyzed by mix-effect models, based on the inverse of the variance, and assessed by heterogeneity tests. The SPSS statistical package (version 15.00) was used. All reported p values were 2-sided, and a p value <0.05 was considered as statistically significant.
Results
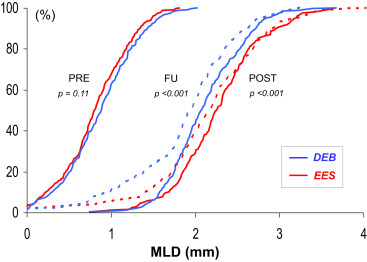
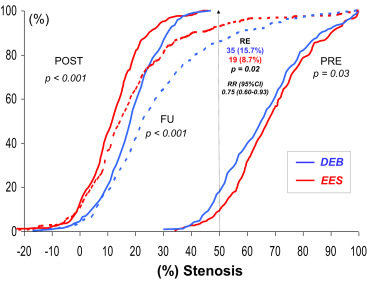
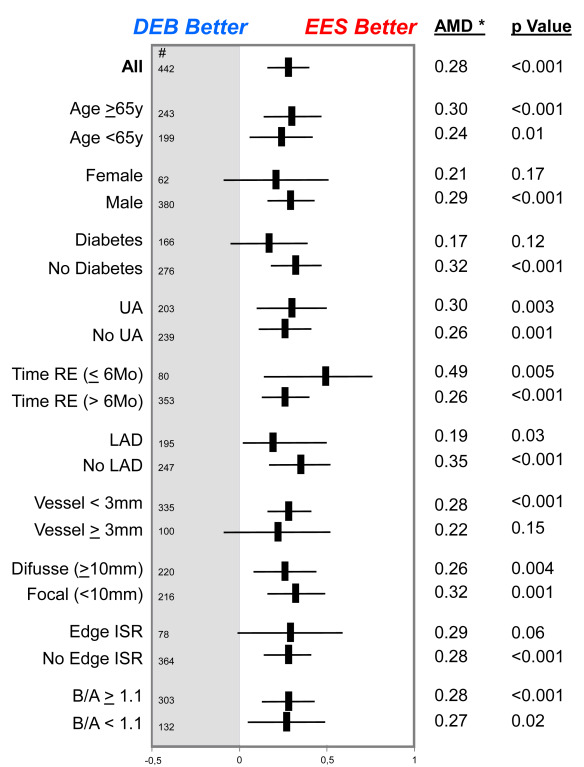
The baseline clinical and angiographic characteristics were nicely balanced in the 2 groups ( Table 1 ). Procedural characteristics were also very similar. High dilation pressures were used in both arms, but significantly higher pressures were used in the EES arm. Angiographic success was obtained in all patients (100%). However, crossover was required in 13 patients (5%) in the DEB group and in 1 patient (0.4%) in the EES group (p <0.01). During hospital stay, 1 patient died from cardiogenic shock after an anterior myocardial infarction and 6 additional patients developed a myocardial infarction. The main angiographic results are summarized in Table 2 . Before the procedure, angiographic characteristics were similar in the 2 groups although lesions in the EES tended to be more severe. However, after intervention, the EES group obtained a significantly larger acute lumen gain and final minimal lumen diameter and a lower residual stenosis. Late angiographic follow-up was obtained in a total of 442 patients (91% of those eligible; median 249 days). The “in-segment” minimal lumen diameter at follow-up (primary end point of the 2 studies) was significantly larger in the EES group (2.16 ± 0.7 vs 1.88 ± 0.6 mm, p <0.001, absolute mean difference 0.28 mm; 95% CI 0.16 to 0.40; Table 2 and Figure 2 ). Importantly, all the remaining major angiographic findings, including % diameter stenosis, net angiographic gain, late loss and loss index, and binary restenosis rate (8.7% vs 15.7%, p = 0.02), were also significantly improved in the EES group ( Table 2 and Figure 3 ). Furthermore, all late angiographic findings were also significantly better in the EES arm when only the in-lesion segment was analyzed ( Table 2 ). There was no apparent heterogeneity in the efficacy of EES versus DEB with respect to the primary end point across the 10 major prespecified subgroups ( Figure 4 ). In patients treated with EES for DES-ISR, no significant differences were found according to the type of underlying DES. Use of intracoronary imaging was not associated with different initial or late angiographic results in the EES or DEB groups.
| Characteristic | DEB (N=249) | EES (N=249) | p Value |
|---|---|---|---|
| Age (years) | 67±10 | 65±10 | 0.07 |
| Women –No (%) | 40 (16%) | 37 (15%) | 0.71 |
| Diabetes mellitus | 105 (42%) | 85 (34%) | 0.07 |
| Insulin-dependent | 35 (14%) | 35 (14%) | 1.00 |
| Hyperlipidemia | 179 (72%) | 183 (74%) | 0.69 |
| Hypertension | 178 (72%) | 189 (76%) | 0.26 |
| Ever smoked | 145 (58%) | 157 (63%) | 0.27 |
| Unstable angina pectoris | 118 (47%) | 121 (49%) | |
| Stable angina/Silent ischemia | 131 (53%) | 128 (51%) | |
| Previous myocardial infarction | 130 (52%) | 133 (53%) | 0.79 |
| Previous coronary bypass | 20 (8%) | 24 (10%) | 0.53 |
| >1 Stent on target lesion | 23 (9%) | 20 (8%) | 0.63 |
| Time to restenosis: days | 496 (240-1639) | 535 (212-1842) | 0.85 |
| Ejection fraction (%) | 58±12 | 59±11 | 0.75 |
| Target Coronary Vessel: | 0.85 | ||
| Left anterior descending | 112 (45%) | 108 (43%) | |
| Left circumflex | 48 (19%) | 56 (23%) | |
| Right | 80 (32%) | 77 (31%) | |
| Saphenous vein graft | 9 (4%) | 8 (3%) | |
| Previous ST Type | 0.93 | ||
| Bare metal stent | 95 (38%) | 94 (38%) | |
| Drug-eluting stent | 154 (62%) | 155 (62%) | |
| Procedural Characteristics: | |||
| Length of the previous stent (mm) | 20±7 | 20±7 | 0.46 |
| Cutting/scoring balloon predilation | 12 (5%) | 9 (4%) | 0.50 |
| Length current EES/DEB (mm) | 20±6 | 21±9 | 0.17 |
| Use of OCT/IVUS | 58 (23%) | 58 (23%) | 1 |
| Maximal pressure (atm) | 18±4 | 20±4 | 0.001 |
| Inflation time (sec) (+) | 108±48 | 62±46 | 0.001 |
| Balloon-to-artery ratio | 1.23±0.2 | 1.19±0.2 | 0.06 |
| Cross-over | 13 (5%) | 1 (0.4%) | 0.001 |
| Angiographic success | 249 (100%) | 249 (100%) | 1 |
| Variable | DEB | EES | p Value |
|---|---|---|---|
| Qualitative Features: | (N=249) | (N=249) | |
| Mehran I,II,III-IV | 135(54%),98(39%),16(7%) | 133(53%),86(35%),30(12%) | 0.08 |
| B2-C lesion | 117 (49%) | 120 (51%) | 0.79 |
| Edge-ISR | 42 (17%) | 46 (19%) | 0.64 |
| Quantitative Findings: | |||
| Before the Procedure: | (N=249) | (N=249) | |
| Reference vessel diameter (mm) | 2.61±0.5 | 2.66±0.5 | 0.32 |
| Minimal lumen diameter (mm) | 0.88±0.4 | 0.82±0.4 | 0.11 |
| Stenosis (% of lumen diameter) | 66±16 | 69±15 | 0.03 |
| Lesion Length (mm) | 11.7±6.5 | 11.9±6.0 | 0.66 |
| Diffuse lesions (>10 mm) | 116 (47%) | 130 (53%) | 0.19 |
| After the Procedure (In Segment) | (N= 249) | (N= 249) | |
| Reference vessel diameter (mm) | 2.62±0.6 | 2.60±0.5 | 0.67 |
| Minimal lumen diameter (mm) | 2.12±0.4 | 2.28±0.5 | <0.001 |
| Stenosis (% of lumen diameter) | 18±11 | 12±11 | <0.001 |
| Acute Gain (mm) | 1.24±0.5 | 1.47±0.5 | <0.001 |
| After the Procedure (In Lesion) | (N= 249) | (N= 249) | |
| Reference vessel diameter (mm) | 2.66±0.5 | 2.72±0.5 | 0.18 |
| Minimal lumen diameter (mm) | 2.20±0.4 | 2.49±0.5 | <0.001 |
| Stenosis (% of lumen diameter) | 17±10 | 8±12 | <0.001 |
| Acute Gain (mm) | 1.31±0.5 | 1.68±0.5 | <0.001 |
| At Follow-up: (In Segment) | (N=223) | (N=219) | |
| Reference vessel diameter (mm) | 2.64±0.5 | 2.69±0.5 | 0.30 |
| Minimal lumen diameter (mm) | 1.88±0.6 | 2.16±0.7 | <0.001 |
| Stenosis (% of lumen diameter) | 28±22 | 19±21 | <0.001 |
| Reestenosis | 35 (15.7%) | 19 (8.7%) | 0.02 |
| Late loss (mm) (mean / median) | 0.24±0.6 / 0.10(-0.16-0.50) | 0.12±0.6 / 0.03(-0.20-0.29) | 0.02 |
| Loss index (mean / median) | 0.16±0.6 / 0.09(-0.14-0.38) | 0.02±0.6 / 0.24(-0.14-0.18) | 0.008 |
| Net gain (mm) | 1.00±0.7 | 1.33±0.6 | <0.001 |
| At Follow-up: (In Lesion) | (N=223) | (N=219) | |
| Reference vessel diameter (mm) | 2.68±0.5 | 2.78±0.5 | 0.06 |
| Minimal lumen diameter (mm) | 1.94±0.6 | 2.30±0.7 | <0.001 |
| Stenosis (% of lumen diameter) | 27±22 | 17±21 | <0.001 |
| Restenosis | 33 (15%) | 17 (8%) | 0.02 |

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


