Previous studies demonstrated that blacks have less coronary artery calcification (CAC) than whites. We evaluated racial differences in plaque composition and stenosis in the Multicenter AIDS Cohort Study. HIV-positive and HIV-negative men underwent noncontrast cardiac computed tomography (CT) if they were aged 40 to 70 years, weighed <136 kg, and had no history of cardiac surgery or revascularization and, if eligible, coronary CT angiography (CTA). There were 1,001 men who underwent CT scans and 759 men CTA. We measured CAC on noncontrast CT and identified total plaque, noncalcified plaque, calcified plaque, mixed plaque, and coronary stenosis >50% on CTA. The association of presence and extent of plaque with race was determined after adjustment for HIV serostatus, cardiovascular risk factors, and measures of socioeconomic status. The prevalences of any plaque on CTA and noncalcified plaque were not different between black and white men; however, black men had lower prevalences of CAC (prevalence ratio [PR] 0.79, p = 0.01), calcified plaque (PR 0.69, p = 0.002), and stenosis >50% (PR 0.59, p = 0.009). There were no associations between black race and extent of plaque in fully adjusted models. Using log-linear regression, black race was associated with a lower extent of any plaque on CTA in HIV-positive men (estimate = −0.24, p = 0.051) but not in HIV-negative men (0.12, p = 0.50, HIV interaction p = 0.005). In conclusion, a lower prevalence of CAC in black compared with white men appears to reflect less calcification of plaque and stenosis rather than a lower overall prevalence of plaque.
It is well established that there are racial differences in coronary artery calcification (CAC), a measure of subclinical atherosclerosis and potent predictor of future coronary events. Despite greater coronary risk factors and cardiovascular morbidity found in blacks, blacks have a paradoxically lower prevalence of CAC and less obstructive coronary artery disease (CAD) compared with whites. It is not known whether the lower prevalence of CAC is secondary to a lower overall prevalence of atherosclerotic plaque or whether it is secondary to a lower proportion of calcified relative to noncalcified plaque for any given plaque volume. Moreover, it is unknown how the presence of human immunodeficiency virus (HIV) infection affects these racial differences. In the Multicenter AIDS Cohort Study (MACS), we previously described that HIV-positive men have a greater prevalence and extent of noncalcified plaque than HIV-negative men. In this report, we evaluated racial differences in CAC, plaque composition, and coronary artery stenosis. We also tested for interactions of HIV serostatus on racial differences in plaque and stenosis.
Methods
Established in 1984, the MACS cohort has enrolled men who have sex with men, both seropositive and seronegative, during 3 enrollment periods from 1984 to 2003 in Baltimore, Chicago, Pittsburgh, and Los Angeles. A cross-sectional cardiovascular study within the MACS enrolled participants from all sites who were aged 40 to 70 years, weighed <136 kg, and had no history of heart surgery or coronary angioplasty. The institutional review boards of all participating sites approved the study.
Participants were seen as part of routine MACS research visits for standardized interviews, physical examination, and blood and urine laboratory collection every 6 months. Data were collected regarding CAD risk factors including age, blood pressure, diabetes and impaired fasting glucose, dyslipidemia, smoking, medication use, body mass index, and HIV clinical parameters. Hypertension was defined as systolic blood pressure >140 mm Hg, diastolic blood pressure >90 mm Hg, or self-reported use of antihypertensive medication. Diabetes mellitus was defined as fasting serum glucose ≥126 mg/dl or use of medications to treat diabetes. Race or ethnicity was based on self-report.
All participants underwent a noncontrast computed tomography (CT) scan for CAC scoring, whereas those with atrial fibrillation, chronic kidney disease (estimated glomerular filtration rate <60 ml/min/m 2 by the Modification of Diet in Renal Disease equation within 30 days), or a contrast allergy were excluded from CT angiography (CTA). Participant heart rates were optimized, and they underwent scanning with electrocardiography-triggered protocols as previously described. In those few men whose heart rates were too fast or irregular, retrospective gating was used.
Noncontrast CT scans were analyzed for CAC using the Agatston method. CTA images were analyzed using the modified 15-segment model of the American Heart Association for plaque presence and extent, coronary artery stenosis, and plaque composition. The total plaque score (TPS) was calculated by summing the plaque size score for all assessable coronary segments that demonstrated any plaque with a maximum score of 45. Calcified atherosclerotic plaque was defined as any structure with attenuation >130 HU visualized separately from the intravascular lumen, identified in at least 2 independent planes. Noncalcified atherosclerotic plaque was defined as any discernible structure that could be clearly assignable to the vessel wall, with a CT density less than the contrast-enhanced coronary lumen but greater than the surrounding connective tissue, and identified in at least 2 independent planes. Finally, mixed plaque included lesions with <50% of plaque area occupied by calcium. The noncalcified plaque score, mixed plaque score, and calcified plaque score were calculated by summing the plaque scores in each segment separately.
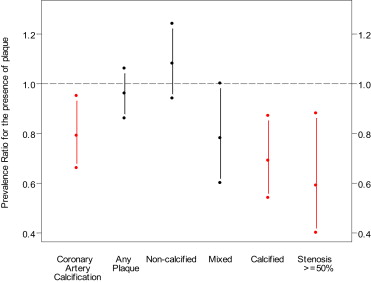
Separate Poisson regressions with robust variance were used to evaluate associations between self-reported race and/or ethnicity and the presence of plaque or stenosis. Presence of plaque was defined as CAC Agatston score >0 from noncontrast CT scans and as TPS, noncalcified plaque score, calcified plaque score, and mixed plaque score >0 on CTA. Coronary stenosis was defined as >50% in any coronary segment. Initial models adjusted for age, HIV serostatus, CT scanning center, and cohort status (enrollment before or after 2001) and included race as a predictor variable with white as the reference group. Additional analyses were then performed with further adjustment for CAD risk factors and education (college vs no college), income, and employment as measures of socioeconomic status (SES). In men with plaque, linear regression was used to assess the extent of plaque present for CAC, TPS, calcified plaque score, mixed plaque score, and noncalcified plaque score in natural log scale using the same models described previously. Analyses were performed in the combined cohort, and a race × HIV interaction term was included in the model to test for differences in the associations between race and plaque between HIV-positive and HIV-negative men. For missing CAD risk factors, multiple imputation was used. Missing values were imputed 5 times based on the distribution of covariates using a Markov chain Monte Carlo method assuming multivariate normality. All statistical analyses were performed using SAS 9.2 (SAS Institute, Cary, North Carolina). Figure 1 was created using TIBCO Spotfire S+ 8.2 (TIBCO Software Inc., Palo Alto, California). Statistical significance was established at a p value of <0.05.
Results
A total of 1,001 noncontrast CT scan and 759 CTA results were available for analysis. Characteristics of the 3 ethnic groups are listed in Table 1 with p values reflecting differences compared with white men. Although not included in the plaque analyses because of small sample size and heterogeneity within the group (Hispanics, Asians, and so on), the category Hispanic/other has been included in the table for complete cohort comparison. There were a total of 581 white men and 305 black men. Black men were younger and more likely to have diabetes, use diabetes medications, to be current smokers, and have more smoking pack-years compared with white men. There were no significant differences between black and white men with respect to hypertension, systolic blood pressure, use of hypertensive medications, or body mass index. Total and low-density lipoprotein cholesterol and triglycerides were lower in black men, whereas high-density lipoprotein cholesterol was higher. Black men were less likely to be taking lipid-lowering medication, to have attended college, to be employed, or have an income >$50,000 per year compared with white men. The prevalence and extent of plaque subtypes stratified by race and/or ethnicity are presented in Table 2 .
| Variable | White | Black | p Value ∗ | Hispanic/Other | p Value † |
|---|---|---|---|---|---|
| N = 581 | N = 305 | N = 115 | |||
| Age (years) | 56.7 (6.7) | 51.6 (6.1) | <0.001 | 49.2 (5.7) | <0.001 |
| Hypertension | 48.1% | 52.1% | 0.27 | 38.1% | 0.05 |
| Systolic blood pressure (mm Hg) | 128.1 (13.7) | 127.7 (16.3) | 0.62 | 121.4 (14.7) | <0.001 |
| Hypertension medications | 33.5% | 36.4% | 0.39 | 31.3% | 0.65 |
| Diabetes mellitus | 9.2% | 14.8% | 0.01 | 13.8% | 0.15 |
| Diabetes medications | 6.1% | 10.6% | 0.02 | 10.4% | 0.09 |
| Tobacco use | |||||
| Never smoked | 29.7% | 17.4% | <0.001 | 23.5% | 0.09 |
| Current smoker | 17.1% | 48.7% | 25.2% | ||
| Former smoker | 53.2% | 33.9% | 51.3% | ||
| Smoking pack-years ‡ | 1.3 (0–26.5) | 8.6 (0.1–22) | 0.002 | 1.2 (0–7.9) | 0.11 |
| Body mass index (kg/m 2 ) | 26.4 (4.5) | 26.9 (5.2) | 0.54 | 26.5 (3.9) | 0.73 |
| Glucose (mg/dL) | 100.1 (±19.9) | 103.9 (±34.4) | 0.89 | 105.2 (±41) | 0.60 |
| Total cholesterol (mg/dL) | 191.7 (±40.6) | 182.5 (±36.8) | 0.003 | 193.9 (±44.1) | 0.68 |
| LDL cholesterol (mg/dL) | 110.5 (±33.7) | 101.7 (±33.4) | <0.001 | 112.3 (±37.6) | 0.90 |
| HDL cholesterol (mg/dL) | 49.2 (±15.3) | 53.5 (±18.9) | 0.002 | 48.5 (±13.4) | 0.71 |
| Triglycerides (mg/dL) | 159.7 (±115.2) | 140.4 (±113.9) | <0.001 | 174.4 (±115.9) | 0.14 |
| Lipid lowering meds | 41.5% | 17.2% | <0.001 | 28.7% | 0.01 |
| Education – college | 64.2% | 24.9% | <0.001 | 40.9% | <0.001 |
| Income (<$20,000) | 20.8% | 64.9% | 45.9% | ||
| Income ($20,000–49,000) | 25.5% | 20.7% | 21.6% | ||
| Income (>$50,000) | 53.7% | 14.4% | <0.001 | 21.6% | <0.001 |
| Employment | 66.8% | 37.8% | <0.001 | 63.5% | <0.001 |
| HIV seropositive | 55.9% | 68.9% | <0.001 | 72.2% | 0.001 |
| HIV clinical factors § | N = 325 | N = 210 | N = 83 | ||
| Undetectable viral load | 90.3% | 70.5% | <0.001 | 81.5% | 0.03 |
| HIV RNA (copies/mL) ‖ | 1440 (104–12200) | 667 (194–34100) | 0.49 | 239 (89–4920) | 0.49 |
| CD4+ T-cell count (cells/mm 3 ) | 600 (436–770) | 597 (405–773) | 0.38 | 628 (477–757) | 0.58 |
| CD4+ T-cell nadir (cells/mm 3 ) | 238 (144–329) | 260 (127–335) | 0.52 | 240 (129–323) | 0.90 |
| On HAART | 97.5% | 92.4% | 0.005 | 98.8% | 0.49 |
| Protease inhibitor use | 47.1% | 48.6% | 0.74 | 55.5% | 0.18 |
| Time on HAART (years) | 13.4 (9.7–14.5) | 10.1 (7.1–13.2) | <0.001 | 12.3 (9.4–12.6) | 0.20 |
| History of clinical AIDS | 17.8% | 10.5% | 0.02 | 9.6% | 0.07 |
∗ p Value comparing white participants to black participants.
† p Value comparing white participants to Hispanic/other participants.
‡ Median (interquartile range: 25% to 75%) for non-normally distributed variables and mean (SD) for normally distributed variables.
‖ Among 107 HIV positive men with detectable HIV RNA (>50 copies/mL) levels.
| CT Scan Parameters | White | Black | Hispanic/Other | Total |
|---|---|---|---|---|
| Non-contrast CT scans (N) | 581 | 305 | 115 | 1001 |
| Coronary artery calcium present | 62.3% | 40.7% | 35.7% | 52.6% |
| Coronary artery calcium score among those with calcium | 82 (25–221) | 49 (17–186) | 41 (24–109) | 71 |
| Contrast-enhanced CT scans (N) | 436 | 230 | 93 | 759 |
| Prevalence of any coronary artery plaque | 82.3% | 70.4% | 62.4% | 76.3% |
| Prevalence of non-calcified plaque | 61.5% | 59.6% | 47.3% | 59.2% |
| Prevalence of mixed plaque | 40.6% | 23.9% | 23.7% | 33.5% |
| Prevalence of calcified plaque | 45.0% | 23.9% | 29.0% | 36.6% |
| Prevalence of any coronary artery stenosis >50% | 21.1% | 8.7% | 9.7% | 12.9% |
| Total plaque score (TPS) | 3 (1–7) | 2 (0–4) | 1 (0–4) | 2 (1–5) |
| Non-calcified plaque score (NCPS) | 1 (0–2) | 1 (0–2) | 0 (0–2) | 1 (0–2) |
| Mixed plaque score (MPS) | 0 (0–2) | 0 (0–0) | 0 (0–0) | 0 (0–1) |
| Calcified plaque score (CPS) | 0 (0–2) | 0 (0–0) | 0 (0–1) | 0 (0–1) |
After adjusting for age, HIV serostatus, study center, and cohort status (minimally adjusted model), black men had a lower prevalence of CAC ( Table 3 ). These results did not change substantially when adjusted for CAD risk factors and measures of SES. In men with CAC present, there was no association between race and extent of CAC. In minimally adjusted models, there was no significant difference in prevalence of any plaque between black and white men, which persisted after adjustment for CAD risk factors and measures of SES. In men with plaque present on CTA, there was a significant inverse association between the extent of any plaque (TPS) and black race, after minimal adjustment. However, after further adjustment for CAD risk factors and SES this association was no longer statistically significant. In addition, there were no differences between black and white men in the extent of any of the plaque subtypes seen on CTA, including noncalcified, calcified, or mixed plaque.
| Prevalence of Plaque | Minimally Adjusted Model | Adjusted for CAD Risk Factors and Socioeconomic Status | ||
|---|---|---|---|---|
| Plaque outcome | Prevalence Ratio (95% CI) | p Value | Prevalence Ratio (95% CI) | p Value |
| Non-contrast CT scans (N = 1001) | ||||
| Coronary artery calcium | 0.76 (0.64–0.90) | 0.002 | 0.79 (0.66–0.95) | 0.01 |
| Contrast CT scans (N = 759) | ||||
| Any plaque | 0.94 (0.84–1.05) | 0.28 | 0.96 (0.86–1.06) | 0.38 |
| Non-calcified plaque | 1.01 (0.87–1.17) ∗ | 0.91 | 1.08 (0.94–1.24) | 0.25 |
| Mixed plaque | 0.74 (0.55–1.00) | 0.047 | 0.78 (0.60–1.00) | 0.054 |
| Calcified plaque | 0.63 (0.48–0.83) | 0.001 | 0.69 (0.54–0.87) | 0.002 |
| Stenosis >50% | 0.48 (0.28–0.81) | 0.006 | 0.59 (0.40–0.88) | 0.009 |
| Extent of Plaque | ||||
|---|---|---|---|---|
| Plaque Outcome | Mean Difference (95% CI) | p Value | Mean Difference (95% CI) | p Value |
| Non-contrast CT | ||||
| Coronary artery calcium (N = 527) | −0.20 | 0.36 | −0.19 | 0.42 |
| Contrast CT scans | ||||
| Total plaque score (N = 579) | −0.23 ∗ | 0.01 | −0.13 ∗ | 0.17 |
| Non-calcified plaque (N = 449) | −0.12 | 0.14 | −0.14 | 0.13 |
| Mixed plaque (N = 254) | −0.14 ∗ | 0.32 | 0.02 | 0.90 |
| Calcified plaque (N = 278) | 0.03 | 0.88 | 0.09 | 0.56 |
When minimally adjusted, black men had a lower prevalence of calcified plaque. This remained statistically significant after adjustment for CAD risk factors and measures of SES. There was a lower prevalence of mixed plaque in black men after minimal adjustment, which became borderline significant after adjustment for CAD risk factors and measures of SES.
There was no association between race and noncalcified plaque after minimal adjustment or after adjustment for CAD risk factors and measures of SES. After adjustment for age, HIV serostatus, study center, and cohort status, black men had a lower prevalence of stenosis >50%, which was not substantially changed after adjustment for CAD risk factors and measures of SES. Figure 1 shows the prevalence ratios with confidence intervals of each plaque type for black men compared with white men from the fully adjusted models.
We evaluated whether associations between black race and coronary atherosclerosis differed by HIV serostatus by including an HIV interaction term in the multivariate model ( Table 4 ). There was a significant HIV interaction for race and extent of any plaque (TPS) on CTA in minimally adjusted model, which persisted after adjustment for CAD risk factors and measures of SES. Using linear regression, there was a borderline association between black race and less overall plaque in HIV-positive men but not in HIV-negative men. There was also a significant HIV interaction for race and presence of noncalcified plaque in the minimally adjusted models, which was borderline statistically significant after adjustment for CAD risk factors and measures of SES. There was a significant HIV interaction for race and extent of mixed plaque on CTA in the minimally adjusted model that did not persist after adjustment for CAD risk factors and measures of SES. Although none of the other HIV interaction tests were statistically significant in the fully adjusted models, there were several borderline significant interactions.



