Impaired left ventricular systolic function (ILVSF) in hypertrophic cardiomyopathy (HC) is a risk factor for sudden death and a determinant of high mortality. We determined its prevalence, clinical parameters, long-term outcome, and pathologic findings of explanted hearts. We retrospectively analyzed 382 patients with HC; ILVSF was characterized by LV ejection fraction <50% at rest and was identified in 24 patients (6.3%). Patients with ILVSF were younger than patients with normal SF (43.5 ± 14.1 vs 55.3 ± 20.4 years, p = 0.001) and had larger LV end-diastolic cavity diameter (53.2 ± 12.2 vs 43.8 ± 6.2 mm, p = 0.001), larger left atrium (51.2 ± 6.5 vs 44.3 ± 8 mm, p <0.001), and lower fractional shortening (30.7 ± 11.1% vs 45.5% ± 10.3%, p <0.001). A combined end point (heart failure death or heart transplantation) was considered. Median follow-up was 3 years (1.2 to 6.3). Fourteen patients with ILVSF (58.3%) had the end point compared to 3 (0.8%) with normal SF (p <0.001). In explanted hearts, fibrosis represented 30.5 ± 12.5% of the left ventricle; we observed a direct correlation between fibrosis and ventricular dilation (r = 0.794, p = 0.001) and an inverse correlation between fibrosis and ejection fraction (r = −0.623, p = 0.023). Number and length density of small arterioles (<50 μm in diameter) were significantly decreased. In conclusion, ILVSF in HC has a poor prognosis and is associated with fibrosis and selective decreased development of small arterioles.
Patients with hypertrophic cardiomyopathy (HC) may exhibit different clinical manifestations ranging from no symptoms to sudden cardiac death or severe heart failure. Impaired left ventricular systolic function (ILVSF) in HC occurs in a small number of patients (about 5%), and the mechanisms involved in its progression are poorly understood. Heart transplantation (HT) is the treatment of choice for patients who remain severely symptomatic despite maximal therapy and for those who progress to heart failure. Our objective was to determine the prevalence and clinical characteristics of patients with ILVSF in a cohort of patients with HC and to evaluate explanted hearts by morphometric methods.
Methods
We reviewed clinical, echocardiographic, and hemodynamic data of 382 patients with HC at our center from June 1992 through September 2009. Diagnosis of HC was suspected from family history and clinical and electrocardiographic findings and confirmed by the presence of typical echocardiographic patterns and/or fiber disarray in endomyocardial biopsy. Electrocardiographic abnormalities included LV hypertrophy, Q waves (duration >0.04 second and/or depth 1/4 of R wave in ≥2 leads), and conspicuous repolarization abnormalities (T-wave inversion in ≥2 leads). Echocardiographic criteria were a hypertrophied left ventricle (wall thickness ≥13 mm) in the absence of any other cardiac or systemic cause of LV hypertrophy. ILVSF in HC was defined as LV ejection fraction <50% at rest by 2-dimensional echocardiography. These criteria were compared between the ILVSF group and normal SF (NSF) group. Events were defined as a combined end point that included death from heart failure or HT.
Thirteen explanted hearts were weighed and fixed in 10% phosphate buffered formaldehyde for 7 days. Time lapse from removal of hearts to fixation was 1 hour to 6 hours. After fixation, sections of the entire circumference of the left ventricle at a plane equidistant from the base to the apex were collected and wall thicknesses were measured at the middle of the interventricular septum, anterior, lateral, and posterior walls of the left ventricle, and free wall of the right ventricle.
For quantitative assessment of fibrosis, a 5-mm-thick slice of the entire left ventricle was collected from the zone equidistant from the apex and the mitral annulus was divided into 8 blocks that were embedded in paraffin, sectioned at 5-μm intervals, and stained with picrosirius red. After obtaining digital images with a digital scanner (UMAX Technologies Inc., Dallas City) at 2× magnification from each tissue block, images were reoriented to reconstruct the entire ventricular transverse section. Percent surface occupied by collagen was established by morphometric analysis using a digital analysis system Image-Pro Plus 4.5 (Media Cybernetics, Silver Spring, Maryland; Figure 1 ).
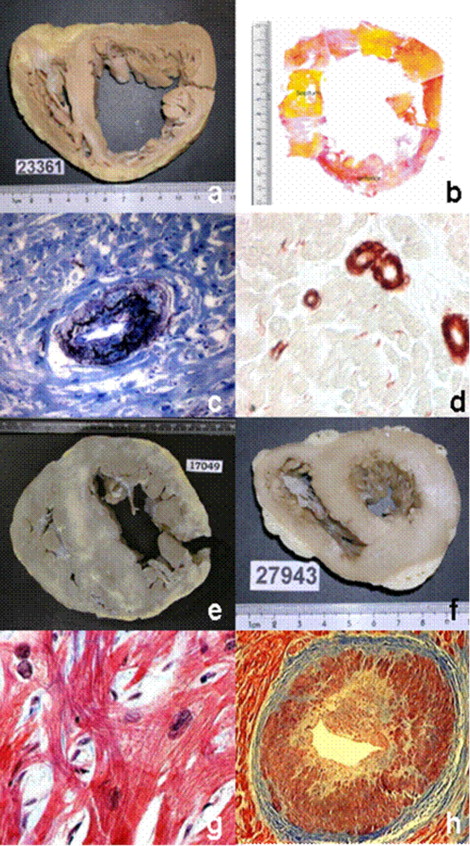
Eight explanted hearts from patients undergoing HT for ILVSF in HC were compared to 6 control hearts collected from beating hearts suitable to provide valves for homografts. For assessment of microcirculation, tissue sections of blocks corresponding to the interventricular septum were stained with orcein to identify the internal elastic lamina of arteries with a diameter of 50 to 250 μm ( Figure 1 ).
For identification of smaller vessels, smooth muscle cells of the middle layer were stained with specific monoclonal antibodies against smooth muscle α-actin (Biogenex, San Ramon, California) ( Figure 1 ). Sections were then treated with biotin-labeled antimouse immunoglobulin antibody and revealed with streptavidin conjugated to peroxidase using AEC (3-amino-9-ethyl carbazole) as chromogen (Biogenex).
For quantitative analysis of small arteries, we considered 3 types of vessels: (1) small arterioles (diameter 6 to 20 μm), (2) medium-size arterioles (diameter 20 to 50 μm), and (3) larger arterioles, (diameter 50 to 250 μm; Figure 1 ). The study was performed using 200× magnification on tissue sections involving the entire midzone of the interventricular septum on a surface from 179.93 to 427.80 mm 2 for vessels >50 μm in diameter and 2,118 mm 2 for smaller vessels 6 to 20 μm in diameter. Number and length densities were determined using Image Pro Plus 4.5.
Numerical density was calculated as number of vessels per square millimeter in the total scanned area. Length density was calculated using a method developed for analysis of vessels arranged in any orientation. For the number of vessels (n) encountered in an area A, length density (LD), expressed in millimeters per unit volume of myocardium (cubic millimeters), is equal to the sum of the ratio (R) of the long to the short axis of each vessel (LD = 1/AΣ[R i = R 1 + R 2 + R 3 … R n ]/A).
Because fibrotic areas presented neovascularization with arteriogenesis, morphometric analysis was performed exclusively on myocardium devoid of fibrosis. In addition, all data were referenced to myocyte area rather than to tissue area. This eliminated any errors caused by shrinkage or separation artifacts and related arterioles to viable at-risk myocytes.
Continuous variables are presented as mean ± SD or median (25% to 75% interquartile ranges), and categorical variables are presented as percentages for all patients. Differences between continuous variables were assessed with Student’s t test or Mann–Whitney test, when applicable. Chi-square or Fisher’s exact test was used to compare categorical variables. Pearson correlation test was applied to analyze the association between fibrosis and clinical, echocardiographic, and morphometric parameters. Univariate Cox proportional hazards regression analyses were used to calculate hazard ratios with 95% confidence intervals for outcomes (stroke, appropriate implantable cardioverter–defibrillator shocks, and death) in patients ILVSF in HC versus NSF. Survival curves were constructed according to the Kaplan–Meier method and were compared using log-rank test. Probability values were significant when <0.05. We used SPSS 11.0 (SPSS, Inc., Chicago, Illinois) for all analyses.
Results
ILVSF was identified in 24 of the 382 patients (6.3%) with HC evaluated at our center. There was no significant difference in follow-up time between the NSF and ILVSF groups (median time to follow-up 3.0 years, interquartile range 1.3 to 6.4, vs 2.2 years, interquartile range 1.2 to 4.8, respectively). Baseline characteristics are listed in Table 1 . Patients who developed ILVSF were younger, had larger LV end-diastolic cavity diameter, larger left atrium, and lower fractional shortening than patients with NSF (p <0.01), and had no obstruction at the time of the study, and only 1 patient had had an obstruction 6 years before development of LV dysfunction. No significant differences were seen in the other clinical variables between the 2 groups, except for that included in the ILVSF election criteria (i.e., LV ejection fraction).
| Characteristic | All Patients | NSF | ILVSF | p Value |
|---|---|---|---|---|
| (n = 382) | (n = 358) | (n = 24) | ||
| Demographic parameters | ||||
| Age (years) | 55 ± 20.2 | 55 ± 20.4 | 44 ± 14.1 | 0.001 |
| Men | 209 (55%) | 195 (55%) | 14 (58%) | 0.713 |
| Family history of hypertrophic cardiomyopathy | 44 (12%) | 40 (11%) | 4 (17%) | 0.503 |
| Symptoms | ||||
| Angina pectoris III to IV (Canadian Cardiovascular Society score for angina) | 18 (5%) | 18 (5%) | 0 (0%) | 0.617 |
| Dyspnea III to IV (New York Heart Association score for heart failure symptoms) | 61 (16%) | 47 (13%) | 14 (58%) | <0.001 |
| Syncope | 46 (12%) | 44 (12%) | 2 (8%) | 0.753 |
| Atrial fibrillation | ||||
| Permanent and paroxysmal | 52 (13%) | 47 (13%) | 5 (20%) | 0.287 |
| Echocardiographic findings | ||||
| Left atrial diameter (mm) | 45 ± 8.0 | 44 ± 8.0 | 51 ± 6.5 | <0.001 |
| Left ventricular end-diastolic diameter (mm) | 45 ± 7.4 | 44 ± 6.2 | 53 ± 12.2 | 0.001 |
| Left ventricular end-systolic diameter (mm) | 25 ± 7.9 | 24 ± 6.1 | 38 ± 13.0 | <0.001 |
| Interventricular septal wall thickness (mm) | 20 ± 6.3 | 20 ± 5.9 | 16 ± 5.7 | 0.005 |
| Left ventricular posterior wall thickness (mm) | 13 ± 3.3 | 13 ± 3.4 | 12 ± 3.3 | 0.638 |
| Fractional shortening (%) | 44 ± 10.7 | 46 ± 10.3 | 31 ± 11.1 | <0.001 |
| Mitral regurgitation, grades 3–4 | 76 (20%) | 73 (20%) | 3 (12%) | 0.438 |
| Basal left ventricular obstruction (≥30 mm Hg) | 144 (38%) | 143 (40%) | 0 (0%) | <0.001 |
| Ejection fraction (%) | 63 ± 10.3 | 65 ± 7.1 | 32 ± 7.4 | <0.001 |
| Risk factors for sudden death | ||||
| Sudden death (documented sustained ventricular tachycardia or ventricular fibrillation) | 9 (3%) | 6 (2%) | 3 (12%) | 0.014 |
| Syncope (documented sustained ventricular tachycardia or ventricular fibrillation) | 6 (2%) | 3 (1%) | 3 (13%) | 0.004 |
| Family history of premature sudden death | 37 (10%) | 33 (9%) | 4 (17%) | 0.273 |
| Massive left ventricular hypertrophy | 34 (9%) | 34 (10%) | 0 (0%) | 0.150 |
| Nonsustained ventricular tachycardia on Holter monitor | 25 (7%) | 22 (6%) | 3 (13%) | 0.201 |
| Unexplained syncope | 20 (5%) | 17 (5%) | 3 (13%) | 0.122 |
| Abnormal blood pressure exercise response | 22 (6%) | 21 (6%) | 1 (4%) | 1.000 |
| 1 major risk factor of sudden death | 40 (11%) | 36 (10%) | 4 (17%) | 0.299 |
| ≥2 major risk factor of sudden death | 45 (12%) | 39 (11%) | 6 (25%) | 0.038 |
Before development of ILVSF, the proportion of patients who received pharmacologic treatment with β blockers, calcium channel antagonists, or a combination did not differ significantly between the 2 groups (disopyramide is not commercially available in Argentina). In the ILVSF group there were more patients on amiodarone than in the NSF group ( Table 2 ). Other major interventions for obstruction or symptoms are listed in Table 2 . During follow-up patients with ILVSF required treatment for systolic dysfunction: angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers (79%), β blockers (75%), diuretics (71%), spironolactone (54%), and warfarin (42%). About 25% of patients with ILVSF treated with maximal medical treatment were clinically stable with compensated heart failure. One responded to cardiac resynchronization therapy with biventricular pacing, whereas 2 other patients did not respond to this treatment.
| All Patients | NSF | ILVSF | p Value | |
|---|---|---|---|---|
| (n = 382) | (n = 358) | (n = 24) | ||
| Medical treatment | ||||
| β Blockers | 241 (63%) | 223 (62%) | 18 (75%) | 0.212 |
| Calcium antagonist | 104 (27%) | 99 (28%) | 5 (21%) | 0.467 |
| β Blockers + calcium antagonist | 54 (14%) | 50 (14%) | 4 (16%) | 0.761 |
| Amiodarone | 35 (9%) | 26 (7%) | 9 (38%) | <0.001 |
| Major interventions for obstruction or symptoms | ||||
| Myectomy | 33 (9%) | 32 (9%) | 1 (4%) | 0.709 |
| Left ventricular percutaneous septal ablation | 5 (2%) | 5 (2%) | 0 (0%) | 1.000 |
| Dual-chamber pacemaker implantation | 17 (5%) | 15 (4%) | 2 (8%) | 0.290 |
| Transplantation | 14 (4%) | 1 (0%) ⁎ | 13 (54%) | <0.001 |
| Implantable cardioverter–defibrillator indication | 52 (14%) | 41 (12%) | 11 (46%) | <0.001 |
| Secondary prevention of sudden death | 5 (1%) | 3 (1%) | 2 (8%) | 0.034 |
| Primary prevention of sudden death | 47 (12%) | 38 (11%) | 9 (38%) | <0.001 |
⁎ This patient underwent transplantation because of surgical complications.
During follow-up 14 patients (58%) with ILVSF reached the combined end point (1 patient [4.2%] died from heart failure and 13 [54%] underwent HT) compared to 3 patients (0.8%) with NSF (p <0.001) ( Table 3 ). One-year and 5-year survivals free from death or HT were 83% and 37%, respectively, in patients with ILVSF versus 98% and 92% in patients with NSF (p <0.001) ( Figure 2 ). Compared to patients with NSF, those who developed ILVSF had a higher frequency of sudden death with documented sustained ventricular tachycardia or ventricular fibrillation and had more syncope with ventricular tachycardia or ventricular fibrillation ( Table 1 ). Three patients with ILVSF waiting for HT underwent appropriate defibrillator interventions ( Table 3 ).



