Assessment of Dual Antiplatelet Therapy With Drug-Eluting Stents (ADAPT-DES) was a prospective, multicenter registry of 8,582 consecutive stable and unstable patients who underwent percutaneous coronary intervention using a drug-eluting stent. We sought to identify key morphologic features leading to ST-segment elevation myocardial infarction (STEMI) versus non-STEMI (NSTEMI) or unstable angina pectoris (UA) versus stable coronary artery disease (CAD) presentation. In the prespecified grayscale and virtual histology (VH) substudy of ADAPT-DES, preintervention imaging identified 676 patients with a single culprit lesion. The relation between lesion morphology and clinical presentation was compared among patients with (1) STEMI, (2) NSTEMI or UA, and (3) stable CAD. Intravascular ultrasound identified more plaque rupture and VH thin-cap fibroatheroma (TCFA) in STEMI lesions compared with NSTEMI/UA or stable CAD lesions; conversely, fibroatheromas appeared more often calcified with a thick fibrous cap in stable CAD. Minimum lumen cross-sectional area (MLA) was smaller with larger plaque burden and positive remodeling in STEMI lesions. Lesions with plaque rupture versus those without plaque rupture showed higher prevalence of VH-TCFA and larger plaque burden with positive remodeling, especially in patients with STEMI. Multivariate analysis showed that in the lesions with plaque rupture, plaque burden at the MLA site was the only independent predictor for STEMI (cutoff of plaque burden = 85%) and in lesions without plaque rupture, MLA was the only independent predictor for STEMI (cutoff of MLA = 2.3 mm 2 ). In conclusion, culprit lesions causing STEMI have smaller lumen areas, greater plaque burden, and more plaque rupture or VH-TCFA compared with NSTEMI/UA or stable CAD; in lesions with plaque rupture, only plaque burden predicted STEMI, and in lesions without plaque rupture, only MLA area predicted STEMI.
Vulnerable plaques have histomorphologic features that are distinct from stable plaques and include a large necrotic core, a thin fibrous cap, and inflammatory cell infiltration. Previous intravascular ultrasound (IVUS) studies have reported features of culprit lesion morphology that cause acute coronary syndrome including plaque rupture, thrombus, larger plaque burden, smaller lumen area, and positive remodeling. In addition, the underlying plaque morphology that causes ST-segment elevation myocardial infarction (STEMI) versus non-STEMI (NSTEMI) has been reported in small studies. The Assessment of Dual Antiplatelet Therapy With Drug-Eluting Stents (ADAPT-DES) study was a large-scale, prospective, multicenter study designed to assess the relation between platelet reactivity and other clinical and procedural variables with subsequent stent thrombosis and adverse clinical events in patients treated with coronary drug-eluting stents (DES). We hypothesized that there was a gradation of plaque vulnerability in relation to clinical presentation. Therefore, the purpose of this substudy was to identify key morphologic features leading to STEMI versus NSTEMI or unstable angina pectoris (UA) versus stable coronary artery disease (CAD) presentation using the data from the prespecified virtual histology (VH)-IVUS substudy cohort.
Methods
In the ADAPT-DES study, consecutive patients at 11 US and German sites successfully treated with 1 or more FDA- or CE mark-approved DES were eligible for enrollment, regardless of patient or lesion complexity. The study has been described in detail previously. The primary end point was definite or probable stent thrombosis according to the Academic Research Consortium definitions. In the prespecified IVUS substudy, preintervention IVUS use was per operator discretion. The study was approved by the institutional review board at each participating center; and all eligible patients signed informed written consent. To evaluate the relation between the clinical presentation and underlying lesion morphology, we included patients with only 1 culprit lesion in a native coronary artery and divided patients into 3 groups according to clinical presentation: (1) STEMI, (2) NSTEMI or UA, and (3) stable CAD.
Angiograms were evaluated visually by operators at the time of the procedure. Thrombus was defined as a discrete intraluminal filling defect with defined borders, largely separated from the adjacent wall and with or without contrast staining. Calcium was defined as readily visible densities noted within the apparent vascular wall at the site of the stenosis. A bifurcation lesion had a branch >1.5 mm in size originating within the stenosis and was completely surrounded by stenotic portions of the lesion to be treated.
Preprocedural grayscale and VH thin-cap fibroatheroma (VH-IVUS) were performed using a synthetic aperture array, 20 MHz, 3.2Fr catheter (Eagle Eye; Volcano Corporation, Rancho Cordova, California) after intracoronary nitroglycerin. The IVUS catheter was advanced distal to the lesion, and the IVUS catheter was pulled backed to the aorto-ostial junction using an R-100 motorized catheter pullback system (0.5 mm/s). During pullback, grayscale IVUS was recorded, raw radiofrequency data were captured at the top of the R-wave, and the reconstruction of the color-coded map by a VH-IVUS data recorder was performed (s5; Volcano Corporation). IVUS studies were archived onto DVD. Off-line grayscale and VH-IVUS analyses of all imaged segments were performed prospectively at an independent core laboratory (Cardiovascular Research Foundation, New York, New York) that was blinded to the clinical events using computerized planimetry software (echoPlaque; INDEC Systems Inc., Mountain View, California).
External elastic membrane and lumen borders were contoured for each slice. Quantitative IVUS measurements included external elastic membrane cross-sectional area (CSA), lumen CSA, plaque and media (external elastic membrane minus lumen) CSA, and plaque burden (plaque and media divided by external elastic membrane). VH-IVUS plaque components were color coded as dense calcium (white), necrotic core (red), fibrofatty (light green), or fibrous tissue (dark green) and reported as CSA and percentages of total plaque CSA. Volumes were calculated using Simpson’s rule and reported as normalized CSA (volume divided by length). The slice with the minimum lumen CSA (MLA) was identified and analyzed. The remodeling index was calculated as the external elastic membrane CSA at the MLA slice divided by the average of the proximal and distal reference external elastic membrane CSA. The distance from the aorto-ostium to the MLA slice or rupture site was calculated using known pullback speed.
Preintervention qualitative grayscale IVUS morphology included plaque rupture (intraplaque cavity that communicated with the lumen with an overlying residual fibrous cap fragment) and calcified nodule (irregular and protruding convex shape of calcium on the calcium plate).
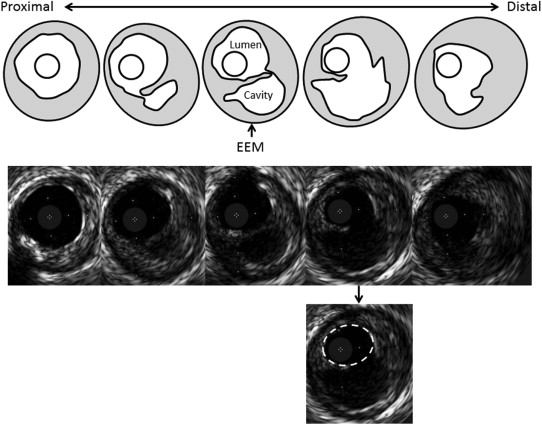
In culprit lesions with plaque rupture, the “expected” pre-rupture lumen CSA was contoured at the maximal plaque rupture frame, and the expected pre-rupture plaque burden was calculated as previously reported ( Figure 1 ). The angle of plaque rupture was also analyzed, and the evacuated cavity CSA at the maximum plaque rupture site was measured.
Using VH-IVUS, lesion phenotype was classified as (1) VH-TCFA, (2) thick-cap fibroatheroma, (3) pathologic intimal thickening, (4) fibrotic plaque, and (5) fibrocalcific plaque. A fibroatheroma (both VH-TCFA and thick-cap fibroatheroma) was defined as >10% confluent necrotic core (spotty red color was not considered as confluent necrotic core). If there was >30° of necrotic core abutting to the lumen in 3 consecutive slices, the fibroatheroma was classified as VH-TCFA; otherwise, it was classified as thick-cap fibroatheroma. Because dense calcium caused shadowing and may have affected VH-IVUS plaque classification, we subcategorized thick-cap fibroatheroma into calcified thick-cap fibroatheroma (dense calcium superficial to necrotic core) and noncalcified thick-cap fibroatheroma (no dense calcium superficial to necrotic core). The VH-IVUS classification was determined both at the MLA slice and within the entire lesion segment. MLA phenotype was based on the morphology at the MLA site. In the entire lesion, the worst phenotype in the lesion was chosen based on the following hierarchy: (1) VH-TCFA, (2) noncalcified thick-cap fibroatheroma, (3) calcified thick-cap fibroatheroma, (4) pathologic intimal thickening, (5) fibrocalcific plaque, or (6) fibrotic plaque.
Categorical variables were compared by the chi-square or Fisher’s exact test. Continuous variables were presented as mean ± SD and were compared by the Student t test or trend analysis for 3 group comparisons. In lesions with versus without plaque rupture separately, candidate IVUS predictors of STEMI (compared with NSTEMI/UA or stable CAD) with p values <0.2 were entered into the multiple logistic regression model. Receiver-operating characteristic C-statistics was used to identify the cut-off value of these independent predictors. A p value <0.05 was considered significant for all analyses. Statistical analyses were performed using SAS, version 9.1.3 (SAS Institute, Cary, North Carolina).
Results
From July 2008 to September 2010, 8,582 patients were enrolled in ADAPT-DES and 2,054 patients were enrolled into a prespecified IVUS substudy. Pre-intervention grayscale and VH-IVUS images in de novo native coronary artery lesions were available in 780 patients with 916 culprit lesions. Among these, 676 patients with a single culprit lesion were finally included in the analysis. In 676 patients with a single culprit lesion and preintervention imaging of that culprit lesion, patient age was 63.4 ± 10.5 years, 24.7% of the patients had diabetes mellitus, 32.5% of the patients were current smokers, and 28.6% of the patients had undergone previous percutaneous intervention ( Table 1 ). Patients with STEMI were younger and more often current smokers compared with those with NSTEMI/UA or stable CAD. Patients with STEMI had fewer coronary risk factors (diabetes mellitus, hypertension, hyperlipidemia, and renal insufficiency) and fewer previous cardiovascular events or revascularizations compared with those with NSTEMI/UA or stable CAD. Patients with STEMI were taking statins less frequently compared with the other 2 groups. Patients with STEMI had more angiographic thrombus and less angiographic calcium compared with the other 2 groups ( Table 2 ).
| Variable | STEMI (n=167) | NSTEMI/Unstable Angina Pectoris (n=217) | Stable Coronary Artery Disease (n=292) | p Value for Trend |
|---|---|---|---|---|
| Age (yrs) | 61.4 ± 10.9 | 63.1 ± 11.0 | 64.7 ± 9.6 | 0.005 |
| Male | 79.0% (132) | 68.7% (149) | 76.0% (222) | 0.05 |
| Body mass index | 27.6 ± 4.7 | 28.3 ± 4.6 | 28.2 ± 5.2 | 0.34 |
| Diabetes mellitus | 16.2% (27) | 24.0% (52) | 30.1% (88) | 0.004 |
| Hypertension | 49.1% (82) | 72.4% (157) | 84.3% (246) | <0.0001 |
| Hyperlipidemia | 34.1% (57) | 49.3% (107) | 70.2% (205) | <0.0001 |
| Current smoking | 43.1% (72) | 35.9% (78) | 24.0% (70) | <0.0001 |
| Renal insufficiency ∗ | 13.8% (23) | 15.2% (33) | 18.8% (55) | 0.31 |
| Prior myocardial infarction | 5.4% (9) | 17.1% (37) | 22.3% (65) | <0.0001 |
| Prior coronary artery bypass grafting | 3.0% (5) | 4.6% (10) | 10.3% (30) | 0.004 |
| Prior percutaneous coronary intervention | 9.6% (16) | 27.2% (59) | 40.4% (118) | <0.0001 |
| Pre-admission medication | ||||
| Aspirin | 76.1% (127) | 74.2% (161) | 67.8% (198) | 0.11 |
| Statin | 19.2% (32) | 38.3% (83) | 61.0% (178) | <0.0001 |
∗ Creatinine clearance <60 mL/min calculated with the Cockcroft-Gault formula.
| Variable | STEMI (n=167) | NSTEMI/Unstable Angina Pectoris (n=217) | Stable Coronary Artery Disease (n=292) | p Value for Trend |
|---|---|---|---|---|
| Three vessels coronary disease ∗ | 21.6% (36) | 24.9% (54) | 26.4% (77) | 0.51 |
| Number of treated lesions | 1.2 ± 0.5 | 1.3 ± 0.7 | 1.4 ± 0.7 | 0.10 |
| Thrombus | 87.4% (146) | 38.7% (84) | 11.0% (32) | <0.0001 |
| Bifurcation lesion | 16.2% (27) | 14.8% (32) | 22.6% (66) | 0.05 |
| Calcium | 25.8% (43) | 50.2% (109) | 43.5% (127) | <0.0001 |
IVUS identified 242 lesions with plaque ruptures in 56.3% of patients with STEMI, 35.5% of NSTEMI/UA, and 24.3% of stable CAD (trend p <0.0001). In addition to more plaque ruptures, culprit lesions ( Table 3 ) in patients with STEMI had more VH-TCFAs at the MLA site (STEMI vs NSTEMI/UA vs stable CAD: 19.8% vs 14.3% vs 8.9%, trend p = 0.0009) and more VH-TCFA as the worst phenotype anywhere within the culprit lesion. Culprit lesions in patients with STEMI had fewer calcified thick-cap fibroatheromas (STEMI vs NSTEMI/UA vs stable CAD: 10.2% vs 19.4% vs 26.4%, trend p <0.0001). Culprit lesions were longer, mean external elastic membrane CSAs were greater, MLAs were smaller with a larger plaque burden with positive remodeling, and arcs of superficial calcium at the MLA sites were smaller in STEMI culprits compared with the other 2 groups. After adjustment of clinical background, STEMI presentation was an independent predictor of plaque rupture (odds ratio [95% CI] = 1.5 [1.0, 2.1], p = 0.06) and VH-TCFA (odds ratio [95% CI] = 1.8 [1.1, 2.7], p = 0.01). The expected pre-rupture lumen area at the rupture site measured 3.7 ± 1.7 mm 2 ; this was larger than the actual MLA in 79.3% and was located proximally compared with the actual MLA site in 65.3%. The angle of plaque rupture measured 106 ± 49°, and the evacuated cavity CSA at the maximum rupture site was 2.2 ± 1.7 mm 2 .
| Variable | STEMI (n=167) | NSTEMI/Unstable Angina Pectoris (n=217) | Stable Coronary Artery Disease (n=292) | p Value for Trend |
|---|---|---|---|---|
| Morphological analysis | ||||
| Plaque rupture | 56.3% (94) | 35.5% (77) | 24.3% (71) | <0.0001 |
| Multiple plaque rupture | 8.4% (14) | 4.1% (9) | 2.4% (7) | 0.004 |
| Calcified nodule | 3.6% (6) | 5.5% (12) | 6.8% (20) | 0.15 |
| VH-TCFA anywhere in the lesion | 65.3% (109) | 52.5% (114) | 44.2% (129) | <0.0001 |
| VH-TCFA at MLA | 19.8% (33) | 14.3% (31) | 8.9% (26) | 0.0009 |
| Calcified ThCFA at MLA | 10.2% (17) | 19.4% (42) | 26.4% (77) | <0.0001 |
| Non-calcified ThCFA at MLA | 42.5% (71) | 39.6% (86) | 36.6% (107) | 0.21 |
| Pathological intimal thickening at MLA | 27.5% (46) | 25.8% (56) | 26.4% (77) | 0.82 |
| Volumetric analysis | ||||
| Lesion length (mm) | 33.6±17.7 | 28.9±16.8 | 28.3±15.9 | 0.004 |
| Mean external elastic membrane CSA (mm 3 /mm) | 15.3±5.7 | 14.1±5.1 | 13.6±5.1 | 0.002 |
| % Plaque volume | 60.9±8.79 | 57.7±8.27 | 55.4±9.2 | <0.0001 |
| % Necrotic core volume | 23.6±8.4 | 22.7±8.0 | 22.0±7.1 | 0.035 |
| % Dense calcium volume | 8.7±5.4 | 10.7±7.4 | 11.6±7.9 | 0.001 |
| Maximum superficial calcium (°) | 111±77 | 114±86 | 116±85 | 0.87 |
| MLA site analysis | ||||
| Lumen cross sectional area (mm 2 ) | 2.5±0.7 | 2.8±1.0 | 3.0±1.1 | <0.0001 |
| External elastic membrane cross sectional area (mm ) | 15.6±6.3 | 13.8±5.8 | 12.9±5.6 | <0.0001 |
| Plaque burden (%) | 80.3±12.3 | 76.5±10.1 | 74.0±10.5 | <0.0001 |
| Remodeling index | 1.11±0.47 | 1.02±0.36 | 0.99±0.41 | 0.0002 |
| Superficial calcium (°) | 35±61 | 46±65 | 55±75 | 0.0014 |
Qualitative and quantitative analysis showed significant differences in lesion morphology between plaque ruptures versus nonruptures including a higher prevalence of VH-TCFA and larger plaque burden with positive remodeling at the MLA site in plaque ruptures compared with nonruptures, especially in patients with STEMI ( Table 4 ).



