Disturbed, nonlaminar flow distal to arterial bifurcations contributes to atherosclerosis development and progression. We hypothesized that the presence of a ramus intermedius (RI) amplifies the flow disturbances in the proximal left anterior descending (LAD) artery causing more proximal LAD lesions and larger ST-segment elevation myocardial infarction (STEMI). Emory University’s contribution to the National Cardiovascular Data Registry was queried for STEMIs from January 2006 to July 2008. The distance from the LAD ostium to the lesion was measured in patients with angiographically visible culprit lesions. The peak troponin-I, creatinine kinase-MB, and left ventricular ejection fraction were used as markers for infarct size. Of the 386 patients with STEMI, 150 had LAD culprit lesions. The mean lesion distance from the LAD ostium was 15.2 ± 11.0 mm in the patients with RI (n = 44) and 29 ± 19 mm in those without RI (n = 106; p <0.01). LAD lesions were more proximal in the patients with RI, with 43% and 63% of lesions occurring in the first 10 and 20 mm of the LAD, respectively, versus 10% and 32% in those without RI (p <0.01). Patients with RI had greater peak troponin-I (69 ± 40 ng/ml vs 50 ± 39 ng/ml, p = 0.01) and peak creatinine kinase-MB (277 ± 271 ng/ml vs 174 ± 190 ng/ml, p = 0.01). A trend was seen toward a lower left ventricular ejection fraction in patients with RI (36 ± 10% versus 40 ± 11%, p = 0.06). In conclusion, the presence of RI was associated with more proximal LAD lesions and larger anterior infarctions, suggesting anatomy-induced flow disturbances have important clinical implications.
Acute ST-elevation myocardial infarction (STEMI) remains a major cause of morbidity and mortality worldwide. Patients with anterior STEMI are at greater risk of in-hospital death, recurrent STEMI, congestive heart failure, stroke, and 1-year mortality than patients with inferior or lateral STEMI. Of the patients with anterior wall STEMI, those with more proximal occlusions in the left anterior descending (LAD) coronary artery have larger myocardial infarctions and worse outcomes. Focal differences in endothelial wall shear stress, in-plaque stress, and wall tension have been linked to flow disturbances. Computational fluid dynamic models have demonstrated that disturbed flow is prevalent in the outer hips of bifurcations, inner curvatures of arteries, and in segments immediately distal to large trifurcations. Whether certain specific anatomic features such as trifurcation of the left main coronary artery result in clinically evident atheroma development and progression in the very proximal LAD is not known. We hypothesized that among patients with anterior wall STEMI, those with a trifurcation of the left main artery owing to the presence of a ramus intermedius (RI) artery would have more proximal LAD culprit lesions than patients without a RI artery.
Methods
Emory University’s contribution to the American College of Cardiology’s National Cardiovascular Data Registry (NCDR) database, a national registry of diagnostic cardiac catheterization and percutaneous coronary interventions, was queried for patients with STEMI. All patients >18 years old, admitted or transferred to Emory University Hospitals for STEMI and emergent cardiac catheterization from January 2006 to July 2008 were included in the NCDR. The diagnosis was confirmed by chart review, including the presenting electrocardiogram and the serum cardiac enzymes. Angiograms of all patients were reviewed to identify the culprit lesion artery. The definition of an infarct-related culprit lesion was prespecified as the site of acute coronary occlusion or, for nonoccluded arteries, as the site of greatest narrowing within an angiographically significant stenosis corresponding to the electrocardiographic changes. Patients were excluded if no clinical evidence for STEMI was found on chart review, no angiograms were available for review, no angiographically identifiable culprit lesion was found, or if the culprit lesion was located in a coronary artery bypass graft or previously placed stent. The angiograms were interpreted by 2 physicians unaware of the patient data, and discordant data regarding the culprit lesion location were resolved by consensus. The demographic and laboratory data were also retrieved from the NCDR database. The peak troponin-I (TN-I), creatinine kinase-MB (CK-MB), and left ventricular ejection fraction (LVEF) were used as markers for infarct size.
Quantitative coronary angiographic analysis of the culprit lesion was performed using a standard software package (QAngio XA, version 7.1.27.0, Medis, Leiden, The Netherlands), and previously published quantitative coronary angiographic techniques for calibration and measurement of the coronary dimensions were used. A standardized angiographic right anterior oblique projection with caudal angulation was chosen for the measurement of each arterial segment within the infarct-related artery to minimize foreshortening. The coronary artery map from the Bypass Angioplasty Revascularization Investigation modification of the Coronary Artery Surgery Study was used for vessel classification.
Quantitative angiographic measurements were performed to identify the distance from the LAD ostium to the culprit lesion. The presence or absence of a RI with an ostial diameter of ≥1 mm was noted.
The clinical characteristics and clinical variables, including patient demographics, clinical history, procedural information, and adverse events that occurred up to hospital discharge, were obtained from the NCDR database. In brief, these data were abstracted using a standard set of 143 data elements from the patient charts, electronic records, and cardiac catheterization laboratory logs by trained research nurses and overseen by an on-site data manger. The elements had written definitions and were gathered using common specifications for all participants. The data were stored at Emory University using commercial software programs precertified by the American College of Cardiology–NCDR. Data authenticity was verified and data auditing performed by the NCDR central data warehouse. Quarterly data sets received at the American College of Cardiology–NCDR passed through quality-control checks to identify missing or improperly coded items. To be acceptable, each quarterly submission had to meet the quality measures for accuracy and completeness. Rejected quarterly data sets could be resubmitted by an institution after correction and could then requalify for inclusion if they passed.
Continuously distributed variables are presented as the mean ± SD, unless otherwise specified. Categorical variables are presented by their frequencies in percentages. For all comparisons, continuously distributed variables were tested for differences using unadjusted analysis of variance or t tests, and categorical variables were tested using Fisher’s exact test. p values <0.05 were considered significant.
Results
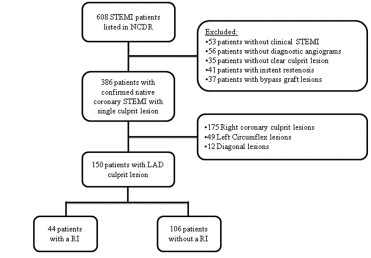
Of the 3,644 patients undergoing a diagnostic catheterization procedure included in the NCDR from the Emory University Hospitals from January 2006 to July 2008, 608 were reported to have presented with STEMI ( Figure 1 ). On chart review, 53 patients were found to not have had STEMI according to the electrocardiographic findings, cardiac enzyme levels, or clinical history. In addition, 169 patients were excluded because of unavailable angiograms before percutaneous coronary intervention (emergent transfers from outside hospitals), the lack of a single identifiable culprit lesion fulfilling the prespecified definition, or if the culprit lesion was located in a bypass graft or previously placed stent. Of the remaining 386 patients with STEMI with angiographically identifiable native coronary culprit lesions, 150 had LAD culprit lesions and constituted the study cohort. Of these 150 patients, a RI was present in 44 (29%) and not in 106 (71%).
The mean age of the study cohort was 59 ± 14 years and 71% were men. The prevalence of a previous myocardial infarction was 8%, and 21% had diabetes mellitus. No significant differences were found in the baseline characteristics between the patients with and without a RI, except for a greater incidence of previous percutaneous coronary intervention in patients with a RI (16% vs 5%, p = 0.04) and a greater likelihood of previous statin use in patients without a RI (28% vs 10%, p = 0.02; Table 1 ).
| Characteristic | Total (n = 150) | Patients With RI (n = 44) | Patients Without RI (n = 106) | p Value |
|---|---|---|---|---|
| Age (years) | 59 ± 14 | 60 ± 12 | 58 ± 14 | 0.41 |
| Men | 107 (71%) | 34 (77%) | 73 (69%) | 0.33 |
| White | 78 (52%) | 25 (57%) | 53 (50%) | 0.48 |
| Smoking | ||||
| Current | 56 (37%) | 15 (34%) | 41 (39%) | 0.71 |
| Former | 24 (16%) | 7 (16%) | 17 (16%) | 1.00 |
| Never | 70 (47%) | 22 (50%) | 48 (45%) | 0.72 |
| Diabetes mellitus | 31 (21%) | 7 (16%) | 24 (23%) | 0.51 |
| Dyslipidemia ⁎ | 85 (57%) | 25 (5%) | 60 (57%) | 1.00 |
| Hypertension | 101 (67%) | 27 (61%) | 74 (70%) | 0.34 |
| Previous percutaneous intervention | 12 (8%) | 7 (16%) | 5 (5%) | 0.04 |
| Previous myocardial infarction (>7 days) | 25 (17%) | 7 (16%) | 18 (17%) | 1.00 |
| Heart failure | ||||
| Current | 26 (17%) | 5 (11%) | 21 (20%) | 0.24 |
| Previous | 8 (5%) | 3 (7%) | 5 (5%) | 0.69 |
| Cardiogenic shock on presentation | 16 (11%) | 7 (16%) | 9 (9%) | 0.24 |
| Ejection fraction during hospitalization (%) | 37.9 ± 10.6 | 35.8 ± 10.2 | 39.9 ± 11.0 | 0.05 |
| Blood products given during hospitalization | 28 (19%) | 12 (27%) | 16 (16%) | 0.11 |
| Medications given during hospitalization | ||||
| Aspirin | 117 (79%) | 35 (80%) | 82 (78%) | 1.00 |
| Clopidogrel | 107 (72%) | 28 (64%) | 79 (76%) | 0.16 |
| β Blocker | 66 (46%) | 18 (42%) | 48 (47%) | 0.59 |
| Heparin | 125 (83%) | 38 (86%) | 87 (82%) | 0.63 |
| Bivalirudin | 23 (16%) | 8 (19%) | 15 (15%) | 0.62 |
| Abciximab | 20 (14%) | 5 (12%) | 15 (15%) | 0.79 |
| Eptifibatide | 51 (35%) | 18 (42%) | 33 (32%) | 0.26 |
| Thrombolytic | 30 (21%) | 6 (14%) | 24 (24%) | 0.19 |
| Statin | 32 (22%) | 4 (10%) | 28 (28%) | 0.02 |
| Bifurcation lesion | 14 (12%) | 3 (9%) | 11 (13%) | 0.76 |
| Complete postprocedure Thrombolysis In Myocardial Infarction flow | 116 (95%) | 30 (88%) | 86 (98%) | 0.05 |
| Intra-aortic balloon pump | 23 (16%) | 9 (21%) | 14 (14%) | 0.32 |
| In-hospital death | 7 (5%) | 3 (7%) | 4 (4%) | 0.42 |
⁎ Total cholesterol >100 mg/dl or low-density lipoprotein ≥130 mg/dl or high-density lipoprotein <40 mg/dl.
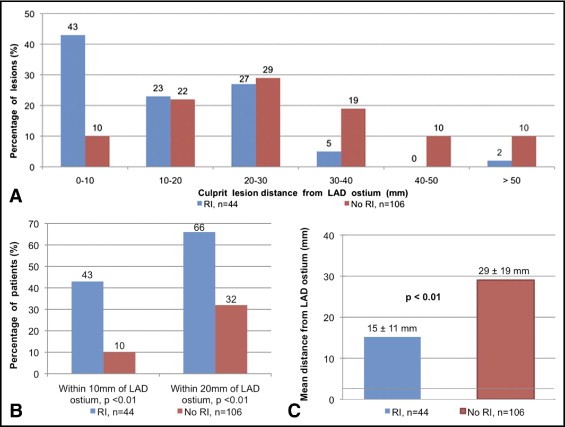
In the study cohort, 130 (85%) of the 150 LAD culprit lesions were within the proximal 40 mm of the artery ( Figure 2 ). Compared with patients without a RI, the culprit lesions in patients with a RI were more commonly located within 10 mm (43% vs 22%, p <0.01) and 20 mm (66% vs 32%, p = 0.01) of the LAD ostium. The mean distance from the LAD ostium to the LAD culprit lesion was 15.2 ± 11.0 mm in patients with a RI and 29.0 ± 19.0 mm without a RI (p <0.01).