The correlation between cardiac computed tomographic (CT) and intravascular ultrasound (IVUS) assessment of saphenous vein graft (SVG) lesions has not been studied. The aim of this study was to evaluate the accuracy of dual-source computed tomography in quantitative assessment of significant SVG lesions scheduled for percutaneous coronary intervention (PCI). Preintervention dual-source CT (DSCT) scans were performed in consecutive patients before PCI of the SVG lesion. All subjects underwent IVUS examination of the target lesion before stent implantation. Lesion characteristics were described using dual-source computed tomography, quantitative coronary angiography, IVUS, and visual estimation. Luminal areas and diameters, lesion lengths, and DSCT suggested stent dimensions were compared. Twenty-two SVG lesions were assessed in 22 patients. Minimal lumen area measured by IVUS was larger than by dual-source computed tomography (3.5 ± 1.2 vs 3.0 ± 1.2 mm 2 , p = 0.04), although there was close correlation between measurements (R = 0.7, p = 0.007). Proximal and distal reference lumen diameters by IVUS and dual-source computed tomography were similar (3.3 ± 0.4 vs 3.4 ± 0.6 mm, p = 0.5, and 3.4 ± 0.6 vs 3.5 ± 0.6 mm, p = 0.4, respectively) and were well correlated (R = 0.85, p <0.0001, and R = 0.81, p <0.0001, respectively). Lesion length by IVUS averaged 18.3 ± 6.1 versus 17.6 ± 5.3 mm by dual-source computed tomography (p = 0.1). There was good correlation between mean reference lumen diameter by dual-source computed tomography and diameter of the implanted stent (R = 0.84, p = 0.0009) and a very good correlation between stent length and lesion length as assessed by dual-source computed tomography (R = 0.9, p <0.0001). In conclusion, DSCT measurements in SVGs correlate with IVUS so that DSCT scan data before PCI of an SVG lesion may be helpful in stent size selection.
Although dual-source computed tomographic (DSCT) spatial and temporal resolutions are much lower than for intravascular ultrasound (IVUS), there has been good correlation between vessel dimensions measured by IVUS and computed tomography in native coronary arteries. Saphenous vein grafts (SVGs) are well visualized using computed tomography including dual-source computed tomography because of their large vessel diameters and relative lack of motion. However, thus far there are no data comparing IVUS with DSCT assessment of SVG lesions. The aims of this study were to compare SVG lesion dimensions before percutaneous coronary intervention (PCI) measured by IVUS, quantitative coronary angiography (QCA), and dual-source computed tomography and to assess if DSCT derived data may be of potential benefit before PCI in SVG.
Methods
The study was approved by the local ethics committee and was performed in accordance with the Second Declaration of Helsinki. All patients participating in the trial signed informed consent. We screened all consecutive patients with significant SVG lesions diagnosed by coronary angiography that were referred for elective PCI. Subjects with renal insufficiency (estimated glomerular filtration rate <60 ml/min/1.73m , atrial fibrillation, and acute coronary syndrome were excluded. All eligible patients underwent DSCT examination before PCI.
DSCT data were acquired using a DSCT scanner (Somatom Definition, Siemens, Forchheim, Germany). All DSCT studies were performed after sublingual administration of nitrates (0.8 mg). In patients with a heart rate >65 beats/min, an additional intravenous bolus of metoprolol (sequential doses of 2.5 mg, maximal dose 10 mg) was given. An electrocardiogram-gated retrospective acquisition protocol was used in all patients with 330-ms rotation time, 0.6-mm collimation, and 80- to 140-kV tube voltage adjusted manually for body mass index. Scan data were reconstructed routinely in mid- to end-diastole (60% to 70% of RR interval). Datasets containing unsatisfactory motion quality were individually optimized by changing the reconstruction window. All DSCT images were stored on digital media for off-line analysis. Off-line DSCT data were analyzed with quantitative CT software (Circulation, Siemens). All measurements were made on cross-sectional arterial reconstructions independently by 2 experienced readers (C.K. and J.P.). References were the most normal-looking segments located within 5 mm from the lesion site. Lumen areas at the lesion site (DSCT minimum lumen area) and proximal and distal reference sites were automatically measured and manually corrected (subjective assessment of independent observers) if necessary. Lumen diameters were measured at these same 3 points: minimal lumen area site and proximal and distal reference sites (minimal lumen diameter, proximal reference lumen diameter, and distal reference lumen diameter). Lesion length was the distance between proximal and distal reference sites.
Before PCI multiple oblique views of the target lesion were acquired using a Siemens angiograph (AXIOM Artus DFC, Forchheim, Germany). The references were the most normal-looking segments located within 5 mm from the lesion site. Angiograms were analyzed independently by 2 experienced observers (J.P. and L.K.) according to clinical standards. Data from visual estimation and QCA were recorded and used for analyses. QCA was performed with a computer-assisted quantitative coronary arteriographic edge-detection algorithm (ACOMPC, Siemens). Contrast-filled 6Fr catheters were used for calibration. End-diastolic frames were chosen for assessment of minimal lumen diameter with QCA and lumen diameters with QCA and visual estimation at the proximal and distal reference sites. Lesion length was the distance between proximal and distal reference sites.
IVUS was performed before PCI after introduction of a distal protection system (FilterWire EZ, Boston Scientific, Natick, Massachusetts) to avoid embolization associated with fragile graft atheroma. Before all IVUS studies intragraft nitroglycerin was administered. A commercially available IVUS catheter (Volcano Corporation, San Diego, California) was advanced distally to the lesion, and imaging was performed retrograde to the aorto-ostial junction with an automatic pullback (0.5 mm/s). Qualitative and quantitative analyses were performed independently by 2 experienced observers (J.P. and L.K.) according to criteria of the American College of Cardiology clinical expert consensus document on IVUS. References were sections with the largest lumen and the least plaque within 5 mm proximal and distal to the lesion. Lumen area was assessed at the minimum lumen area lesion site and proximal and distal reference sites. Lumen diameters were measured at these same 3 points (minimal lumen diameter, proximal reference lumen diameter, and distal reference lumen diameter). Lesion length was the distance between the proximal and distal reference sites as assessed with automatic pullback.
The operator performing PCI was blinded to DSCT results. The final decision on the diameter and length of the stent selected was at the discretion of the operator. The suggested stent diameter in the present study was the average of proximal and distal reference lumen diameters as measured by IVUS. The suggested stent length was the IVUS pullback length between cross sections proximal and distal to the stenosis site with lumen area ≥5 mm 2 and plaque burden <40%.
Continuous data with normal distribution are presented as mean ± SD and non-normally distributed variables are presented as median with interquartile range. Correlations of continuous variables were assessed with the Spearman test. A 2-tailed paired-sample t test or Wilcoxon test was used to assess differences between continuous variables. Intraclass correlation coefficient (a method of agreement for continuous variables) was used to assess interobserver variability in visually estimated, IVUS, and DSCT measurements. Bland–Altman plots were produced to visualize the difference between measurements by different imaging techniques. MedCalc 9.3.8.0 (MedCalc, Marierkerke, Belgium) was used for statistical analyses.
Results
From May 2008 through September 2010 there were 40 patients who underwent elective PCI for SVG lesions in our hospital. We excluded 5 patients because of atrial fibrillation and 9 patients because of low estimated glomerular filtration rate. Because 4 patients refused to participate in the project, our study group consisted of 22 subjects ( Table 1 ). In all 22 patients DSCT scan was performed before IVUS-guided PCI of the SVG lesions. Median time from cardiac DSCT angiography to PCI was 3 days (interquartile range 1 to 7). IVUS with automatic motorized pullback was performed before intervention in all lesions. Two target lesions were located ostially; therefore, there are no proximal reference measurements for those subjects. In 4 patients CT measurements at the minimal lumen site could not be performed because of high-grade stenosis that led to contrast column disappearance within the lesion.
| Patient | Age (years)/Sex | Graft Age (years) | Artery Supplied by Target Graft | Previous Myocardial Infarction (0/1) | Diabetes Mellitus (0/1) | Hypertension (0/1) | Hypercholesterolemia (0/1) | Active Smoker (0/1) | NYHA Functional Class >II Heart Failure (0/1) |
|---|---|---|---|---|---|---|---|---|---|
| ED | 57/M | 10 | OM, Diag, RCA | 1 | 0 | 1 | 1 | 0 | 0 |
| RW | 57/M | 15 | RCA | 1 | 0 | 1 | 0 | 1 | 1 |
| JS | 58/M | 5 | OM, RCA | 0 | 0 | 1 | 1 | 0 | 0 |
| AS | 60/M | 12 | OM | 0 | 1 | 0 | 1 | 0 | 0 |
| MA | 61/M | 11 | RCA | 1 | 0 | 1 | 1 | 0 | 1 |
| MJ | 61/M | 13 | LAD | 1 | 0 | 0 | 1 | 0 | 0 |
| WK | 61/M | 13 | RCA | 0 | 1 | 1 | 1 | 0 | 0 |
| GJ | 62/F | 15 | LAD | 1 | 1 | 1 | 1 | 0 | 0 |
| JM | 65/M | 16 | OM | 0 | 1 | 1 | 1 | 0 | 0 |
| ZS | 66/M | 1 | OM, LCx | 1 | 1 | 1 | 1 | 0 | 0 |
| WS | 68/M | 20 | RCA | 0 | 0 | 1 | 0 | 0 | 0 |
| JK | 69/M | 12 | LAD | 1 | 1 | 0 | 1 | ||
| ZK | 70/M | 8 | RCA | 1 | 0 | 1 | 1 | 0 | 0 |
| EK | 70/F | 11 | LAD | 1 | 0 | 1 | 0 | 0 | 0 |
| KZ | 75/M | 12 | OM, LCx | 1 | 0 | 1 | 1 | 0 | 1 |
| JG | 75/M | 15 | LAD | 1 | 1 | 0 | 1 | 0 | 0 |
| WM | 76/M | 17 | OM | 0 | 0 | 0 | 1 | 0 | 0 |
| FF | 77/M | 15 | LAD, Diag | 1 | 0 | 1 | 1 | 0 | 1 |
| WP | 77/M | 20 | OM, LCx | 1 | 1 | 1 | 1 | 0 | 0 |
| TN | 78/M | 15 | LAD | 0 | 0 | 0 | 1 | 0 | 0 |
| ZK | 81/F | 11 | LAD | 0 | 0 | 1 | 1 | 0 | 0 |
| WT | 84/F | 4 | RCA | 1 | 0 | 1 | 1 | 0 | 1 |
Minimum lumen area by IVUS was significantly larger than by dual-source computed tomography (3.5 ± 1.2 vs 3.0 ± 1.2 mm 2 , p = 0.04). However, there was a significant correlation between the 2 measurements (R = 0.7, p = 0.007). Also, minimal lumen diameter by IVUS was significantly larger than minimal lumen diameter by dual-source computed tomography (2.0 ± 0.4 vs 1.8 ± 0.3 mm, p = 0.009), although results obtained with the 2 imaging techniques were also significantly correlated (R = 0.51, p = 0.02). Proximal reference lumen area measured by IVUS was smaller than by dual-source computed tomography (8.7 ± 2.4 vs 9.2 ± 2.3 mm 2 , p = 0.02), whereas distal reference lumen area measurements were similar (8.6 ± 2.2 vs 9.0 ± 2.7 mm 2 , p = 0.12). We found good correlations between IVUS and dual-source computed tomography for proximal and distal reference lumen areas (R = 0.86, p <0.0001, and R = 0.90, p <0.001, respectively).
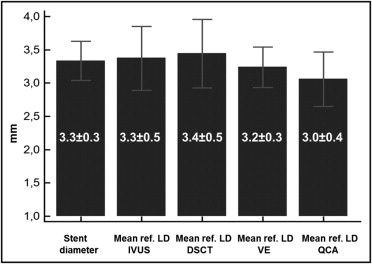
There were no differences in proximal reference lumen diameter between IVUS and dual-source computed tomography (3.3 ± 0.4 vs 3.4 ± 0.4 mm, p = 0.4) or in distal reference lumen diameter between IVUS and dual-source computed tomography (3.4 ± 0.6 vs 3.5 ± 0.6, p = 0.5, mean reference lumen diameters; Figure 1 ). There was good correlation between the 2 methods (R = 0.85, p <0.0001, for proximal reference and R = 0.81, p <0.0001, for distal reference; Figure 2 ). Bland–Altman plots showed systematic errors of 1.1% (mean difference) for the comparison in proximal reference lumen diameter between IVUS and dual-source computed tomography and 1.6% for the comparison in distal reference lumen diameter between IVUS and dual-source computed tomography ( Figure 3 ). An example of the similarity between luminal measurements performed with IVUS and dual-source computed tomography is presented in Figures 4 and 5 . Lesion length by IVUS measured 18.3 ± 6.1 versus 17.6 ± 5.3 mm by dual-source computed tomography (p = 0.1). There was a good correlation in lesion length measurements by IVUS and dual-source computed tomography (R = 0.89, p <0.0001).