Left atrial appendage occlusion (LAAO) is emerging as a promising alternative to oral anticoagulation. Because aged patients present a greater risk of not only cardioembolic events but also major bleeding, LAAO might represent a valid alternative as this would allow oral anticoagulation cessation while keeping cardioembolic protection. The objective of the study was to explore the safety and efficacy of LAAO in elderly patients. Data from the AMPLATZER Cardiac Plug multicenter registry were analyzed. The cohort was categorized in 2 groups (<75 vs ≥75 years). A total of 1,053 subjects were included in the registry. Of them, 219 were excluded because of combined procedures. As a result, 828 subjects were included (54.6% ≥75 years). Procedural success was high and similar in both groups (97.3%). Acute procedural major adverse events were not statistically different among groups (3.2% in <75 years vs 5.1%; p = 0.17) although stratified analysis showed a higher incidence of cardiac tamponade in elderly patients (0.5% vs 2.2%; p = 0.04). With a median follow-up of 16.8 months, no significant differences in stroke/TIA (1.9% vs 2.3%; p = 0.89) and major bleeding (1.7% vs 2.6%; p = 0.54) were observed. In conclusion, LAAO was associated with similar procedural success in patients aged <75 and ≥75 years although older patients had a higher incidence of cardiac tamponade. At follow-up, stroke and major bleeding rates were similar among groups.
Medical management of nonvalvular atrial fibrillation (NVAF) represents an important challenge as aged patients present a greater risk of not only cardioembolic events but also major bleeding. Indeed, the age is one of the shared items for both CHA 2 DS 2 -VASc and HAS-BLED (Hypertension, Abnormal renal/liver function, Stroke, Bleeding history or predisposition, Labile international normalized ratio, Elderly (>65 years), Drugs/alcohol concomitantly) scores. Patients aged ≥75 years and NVAF are clear examples of this challenge as the age by itself (≥75 years) constitutes a solid indication for oral anticoagulation (OAC) but at the same time implies a greater prevalence of additional co-morbidities that might increase the CHA 2 DS 2 -VASc and HAS-BLED scores. Elderly patients might be good candidates for percutaneous left atrial appendage occlusion (LAAO) as this would allow OAC cessation while keeping cardioembolic protection. However, because patients aged ≥75 years are generally more fragile and therefore more prone to complications during interventional procedures, some specific analysis is deemed necessary. The present study tries to clarify this question as it assesses the safety and efficacy of LAAO using a cut-off value of 75 years based on the CHA 2 DS 2 -VASc score threshold.
Methods
The study included consecutive patients with NVAF who underwent LAAO with the AMPLATZER Cardiac Plug (ACP) in 22 centers from December 2008 to November 2013. Data were derived from the multicenter ACP study. For the purpose of our study, the cohort was divided into 2 groups (<75 years vs ≥75 years). To avoid selection bias, patients with combined procedures during LAAO, such as percutaneous coronary interventions, transcatheter aortic valve implantation (TAVI), atrial septal defect occlusion, or others, were excluded from the analysis. Prospectively collected data from each center were transferred to a dedicated database and were analyzed retrospectively. The study was approved by the institutional review committee of every center, and the included subjects gave informed consent. Details regarding LAAO procedure and special features of the ACP device have been published elsewhere.
Procedural success was defined as successful implantation of the ACP in the left atrial appendage (LAA).
Periprocedural adverse events (occurring during 0 to 7 days postprocedure or before hospital discharge, whichever last) was based on the VARC criteria (11) and included death, myocardial infarction, stroke, transient ischemic attack (TIA), systemic embolization, air embolization, device embolization, significant pericardial effusion or cardiac tamponade, and major bleeding (requiring surgery or transfusion). The definition for major adverse events was the following: acute (0 to 7 days) occurrence of death, stroke (ischemic or hemorrhagic), systemic embolism, and procedure or device-related complications requiring major cardiovascular or endovascular intervention. This definition was used to be able to compare the study results with previous reports.
Clinical follow-up included implanted patients and was carried out by patient visits or phone contact, per center or operator protocol. Report of adverse events during follow-up was based on the VARC criteria and included death (cardiovascular or non-cardiovascular), stroke, TIA, systemic embolism, and major bleeding. All centers provided a summary for every reported major adverse event. Antithrombotic medication was recorded at the admission date and at the last follow-up visit. The recommendation by the device manufacturer after LAAO was to prescribe acetylsalicylic acid 80 to 100 mg and clopidogrel 75 mg/day for 1 to 3 months and then only acetylsalicylic acid 80 to 100 mg for at least another 3 months. However, the choice and the duration of antithrombotic therapy were individualized depending on the patient history, indication for LAAO, and physician preference.
Device efficacy to prevent stroke, TIA, and systemic embolism was tested by comparing the actual event rate at follow-up with the predicted event rate by the CHA 2 DS 2 -VASc score. Individual patient annual risk was recorded, and the average annual risk for the whole study population was calculated. The total number of thromboembolic events (as defined by stroke, TIA, and systemic embolism) during both the periprocedural and follow-up periods were divided by the total patient years of follow-up and were multiplied by 100 to get the actual annual rate of thromboembolism. Thromboembolism reduction was calculated as follows: (estimated % − actual % event rate)/estimated % event rate.
Bleeding reduction was assessed in analogy to stroke reduction. The total number of major bleeding events (periprocedural and at follow-up) per year was compared with the events predicted by the HAS-BLED score : (estimated % − actual % event rate)/estimated % event rate.
Implanted patients underwent a transthoracic echocardiography (TEE) at follow-up per center or operator protocol. Color Doppler was used to assess peridevice leaks in multiple views. Leaks were categorized according to the width of the color jet as follows: trivial (<1 mm), mild (1 to 3 mm), or significant (>3 mm). Successful LAAO was defined as the absence of significant (>3 mm) leak at the last TEE. All TEEs underwent interrogation for device thrombosis.
The primary end point was device efficacy to prevent stroke, TIA, and systemic embolism. Secondary end points included bleeding events, cardiovascular mortality, noncardiovascular mortality, and all-cause mortality.
Continuous variables were explored for normal distribution using the Kolmogorov–Smirnov test. Variables following normal distribution were expressed as mean ± standard deviation, and non-normally distributed variables were expressed as median (interquartile range [IQR]). Categorical variables were expressed as count and percentage. Baseline characteristics between groups were compared using t test for continuous variables and the chi-square test for categorical variables. Hazard ratio was estimated using Cox proportional hazards regression model. Survival curves between 2 groups (<75 years vs ≥75 years) were constructed for assessment of end points and compared with the log-rank test. Considering the variable follow-up in patients, survival curves were constructed at 1-year follow-up as it was a meaningful timeline value and to have a sufficient number of patients to perform a valid analysis. Statistical analysis was performed using the STATA version 13 (STATA Corp., Texas).
Results
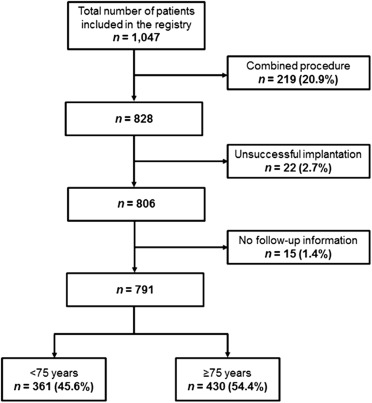
A total of 1,053 subjects were included in the registry. Of them, 219 (20.7%) were excluded from the analysis because of a combined interventional procedure during LAAO, and 6 (0.6%) because of incomplete procedural data. As a result, 828 subjects were included in the analysis and 452 (54.6%) were aged ≥75 years at the time of the procedure ( Figure 1 ).
Baseline characteristics of the study population stratified according to group age are provided in Table 1 . The mean age of the study population was 75.6 ± 7.9 years. Compared to the younger group, patients aged ≥75 years had a lower body mass index, a greater proportion of women and greater CHA 2 DS 2 -VASc and HAS-BLED scores. The increased risk for stroke in elderly patients was secondary to age and a greater proportion of female patients as there were no significant differences between groups in the prevalence of chronic heart failure, hypertension, diabetes mellitus, or previous stroke. Baseline antiplatelet and OAC treatment was similar among groups.
| Variable | Age (years) (n = 828) | P value | |
|---|---|---|---|
| < 75 (n =376) | ≥ 75 (n = 452) | ||
| Age, years | 68.4 ± 5.6 | 81.2 ± 4.1 | <0.01 |
| Body mass index (kg/m 2 ) | 28.2 ± 5.1 | 26.3 ± 4.0 | <0.01 |
| Men | 255 (67.8%) | 248 (54.9%) | <0.01 |
| Chronic heart failure | 99 (26.3%) | 114 (25.2%) | 0.72 |
| Hypertension | 327 (87.0%) | 395 (87.4%) | 0.86 |
| Diabetes mellitus | 123 (32.7%) | 134 (29.8%) | 0.36 |
| Previous TIA / stroke | 150 (39.9%) | 172 (38.1%) | 0.59 |
| CHADS 2 score | 2.4 ± 1.2 | 3.3 ± 1.3 | <0.01 |
| CHA 2 DS 2 -VASc score | 3.9 ± 1.5 | 5.1 ± 1.4 | <0.01 |
| HASBLED | 3.0 ± 1.3 | 3.4 ± 1.2 | <0.01 |
| Atrial fibrillation pattern | |||
| Permanent | 200 (53.2%) | 293 (64.8%) | <0.01 |
| Persistent or paroxysmal | 176 (46.8%) | 158 (34.9%) | <0.01 |
Indication for LAAO was similar in both groups ( Table 2 ). As presented in Table 3 , LAAO was successfully performed in 806 patients (97.3%), without significant differences between groups. Overall, major adverse events were not statistically different among groups, but individual analysis of procedural complications showed a higher incidence of cardiac tamponade in aged patients.
| Variable | Age (years) (n = 828) | P value | |
|---|---|---|---|
| < 75 (n =376) | ≥ 75 (n = 452) | ||
| Indication | |||
| Previous major bleeding | 182 (48.4%) | 215 (47.7%) | 0.83 |
| Thromboembolism on OAC | 50 (13.3%) | 74 (16.4%) | 0.22 |
| Other | 165 (43.9%) | 198 (43.8%) | 0.98 |
| Success of procedure | 366 (97.3%) | 440 (97.3%) | 0.99 |
| Transseptal route | 359 (95.5%) | 440 (97.3%) | 0.15 |
| PFO route | 17 (4.5%) | 12 (2.6%) | 0.15 |
| Variable | Age (years) (n = 828) | P value | |
|---|---|---|---|
| < 75 (n =376) | ≥ 75 (n = 452) | ||
| Death | 1 (0.27%) | 6 (1.33%) | 0.10 |
| Stroke | 4 (1.1%) | 3 (0.7%) | 0.53 |
| Major bleeding | 3 (0.8%) | 6 (1.3%) | 0.46 |
| Device embolization | 2 (0.5%) | 4 (0.9%) | 0.55 |
| Pericardial effusion | 8 (2.1%) | 13 (2.9%) | 0.49 |
| Cardiac tamponade | 2 (0.5%) | 10 (2.2%) | 0.04 |
| Major adverse events | 12 (3.2%) | 23 (5.1%) | 0.17 |
A total of 791 patients were included in the follow-up analysis ( Figure 1 ). Average follow-up was 16.5 months (IQR 17.8 months) in the younger group and 17.2 months (IQR 19 months) in the older one, accumulating a total of 490 and 607 patient-years, respectively. Table 4 provides the adverse events at follow-up. There were no significant differences between groups in stroke/TIA and major bleeding rates. All-cause and noncardiovascular mortality rate was higher in elderly patients, whereas cardiovascular mortality showed no significant differences among groups. Importantly, survival curves at 1 year for all the previous outcomes confirmed the aforementioned results ( Figures 2 and 3 ).



