Abciximab and eptifibatide have been shown to reduce ischemic complications compared with heparin alone in patients with acute coronary syndromes who undergo percutaneous coronary intervention. Whether 1 agent is safer and/or more effective has not been prospectively examined. The aim of this study was to assess the outcomes related to downstream glycoprotein IIb/IIIa inhibitor treatment selection during percutaneous coronary intervention in 2,211 patients with moderate and high-risk acute coronary syndromes in the prospective multicenter Acute Catheterization and Urgent Intervention Triage Strategy (ACUITY) trial. The protocol permitted operator selection of abciximab (n = 835) or eptifibatide (n = 1,376) for routine use in the trial. Multivariate and propensity-based adjustments were used to assess the independent association of glycoprotein IIb/IIIa inhibitor treatment selection with prespecified study end points. Compared to patients receiving eptifibatide, those administered abciximab were older, more likely to be enrolled outside of North America, more frequently had biomarker elevations and ST-segment deviation, but had fewer baseline cardiac risk factors and previous revascularization procedures. After multivariate propensity-based adjustment, abciximab was independently associated with significantly fewer net clinical adverse events (odds ratio 0.61, 95% confidence interval 0.42 to 0.90, p = 0.01), mediated by composite ischemia (odds ratio 0.61, 95% confidence interval 0.38 to 0.98, p = 0.04) and major bleeding (odds ratio 0.58, 95% confidence interval 0.34 to 1.00, p = 0.051). In conclusion, in this prespecified but nonrandomized comparison in patients with acute coronary syndromes who underwent percutaneous coronary intervention with catheterization laboratory initiation of glycoprotein IIb/IIIa inhibitors, the use of abciximab rather than eptifibatide was associated with improved clinical outcomes at 30 days. These findings should be viewed as exploratory in light of the nonrandomized and heterogenous nature of the comparator groups and significant potential for residual confounding.
In patients who undergo percutaneous coronary intervention (PCI), glycoprotein (GP) IIb/IIIa inhibitors reduce adverse myocardial ischemic events compared to anticoagulation with heparin alone. The absolute benefits of GP IIb/IIIa inhibition are greater in patients who undergo PCI with acute coronary syndromes (ACS) than in those with stable ischemic syndromes. In this regard, abciximab and double-bolus eptifibatide administered to heparin-treated patients have been demonstrated in randomized studies to be superior to heparin monotherapy during PCI and are recommended with a class I level of evidence for use during PCI in high-risk patients with ACS who undergo early invasive management strategies. Abciximab is a monoclonal antibody that irreversibly inactivates the platelet GP IIb/IIIa receptor, while eptifibatide is a small-molecule competitive inhibitor of the GP IIb/IIIa receptor with a short half-life. Despite these and other differences, surprisingly few studies have been performed in which these 2 agents have been directly compared. We therefore examined the comparative outcomes of catheterization laboratory–initiated abciximab and eptifibatide in moderate- and high-risk patients with ACS who underwent PCI in the Acute Catheterization and Urgent Intervention Triage Strategy (ACUITY) trial.
Methods
As previously described, ACUITY was a prospective, open-label, randomized, multicenter trial in which patients with moderate- and high-risk ACS who underwent an early invasive strategy were randomized to heparin plus a GP IIb/IIIa inhibitor, bivalirudin plus a GP IIb/IIIa inhibitor, or bivalirudin alone. Randomization was stratified by site and by the use or intent to administer a thienopyridine before angiography. Patients were randomized to 1 of 3 antithrombin or antiplatelet strategies: heparin (either unfractionated or enoxaparin at site discretion) plus a GP IIb/IIIa inhibitor, bivalirudin plus a GP IIb/IIIa inhibitor, or bivalirudin alone. Patients assigned to heparin plus a GP IIb/IIIa inhibitor or bivalirudin plus a GP IIb/IIIa inhibitor were randomized again in a 2 × 2 factorial design to routine upstream GP IIb/IIIa inhibitor initiation in all patients immediately after randomization versus deferred selective GP IIb/IIIa inhibitor initiation in the catheterization laboratory for patients in whom diagnostic angiography identified anatomy or lesions suitable immediate PCI. Angiography was performed by protocol <72 hours after randomization, after which the decision was declared and recorded for primary treatment either with PCI, coronary artery bypass grafting (CABG), or medical management.
Antithrombin agents were administered per standard dosing regimens. Aspirin was administered daily during hospitalization. The initial dosing and timing of clopidogrel were left to investigator discretion per local standards, although a loading dose of ≥300 mg was required no later than 2 hours after PCI in all patients. According to current guideline recommendations, for initiation in the catheterization laboratory, either abciximab (0.25 mg/kg bolus plus 0.125 μg/kg/min infusion, maximum 10 μg/min) or eptifibatide (2 boluses of 180 μg/kg 10 minutes apart, plus a 2.0 μg/kg/min infusion), was permitted at the investigator’s choice, begun 5 to 10 minutes before the first balloon inflation. GP IIb/IIIa inhibitors were continued for 12 hours (abciximab) or 12 to 18 hours (eptifibatide) after PCI.
The 3 primary 30-day end points prespecified in the ACUITY trial were also used for this substudy: (1) composite ischemia, defined as death from any cause, myocardial infarction, or unplanned revascularization for ischemia; (2) major bleeding (non-CABG related), defined as intracranial or intraocular bleeding, access-site hemorrhage requiring intervention, ≥5-cm-diameter hematoma, reduction in hemoglobin ≥4 g/dl without or ≥3 g/dl with an overt bleeding source, operation for bleeding, or blood product transfusion; and (3) net adverse clinical events (NACEs; composite ischemia or major bleeding). All primary end points were adjudicated by a blinded clinical events committee.
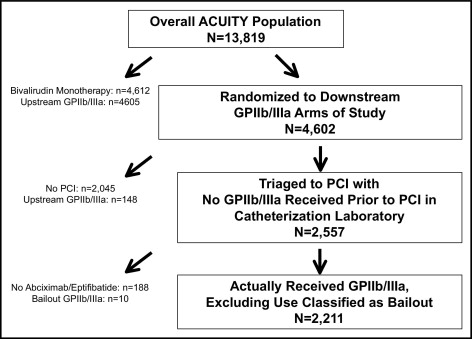
The present report represents a prespecified substudy of the ACUITY trial. For the purposes of this analysis, only patients who underwent PCI and were randomized to “downstream” or catheterization laboratory initiation of GP IIb/IIIa inhibitors were included. Patients receiving upstream GP IIb/IIIa inhibitors (either through randomization or as bailout therapy), patients who did not receive either eptifibatide or abciximab in the catheterization laboratory, and patients randomized to bivalirudin monotherapy who received bailout GP IIb/IIIa inhibitors were excluded from this analysis. The primary study analysis was that of abciximab versus eptifibatide initiated in the catheterization laboratory in patients with ACS who underwent PCI, a comparison that was nonrandomized. Furthermore, because there were no significant differences in the overall ACUITY population in any of the 3 primary end points between patients randomized to heparin plus a GP IIb/IIIa inhibitor and those randomized to bivalirudin plus a GP IIb/IIIa inhibitor, the primary analysis population for this substudy was pooled from all patients regardless of antithrombin use. Formal interaction testing was performed to determine whether the results were dependent on the antithrombin randomization.
Categorical variables were compared using chi-square tests and are presented as frequencies and percentages. Continuous variables were compared using Student’s t test and are presented as mean ± SD or as medians with interquartile ranges. A 2-sided α value <0.05 was used to assess statistical significance. Because the primary study comparison (abciximab vs eptifibatide) was nonrandomized, multivariate models were used to adjust for potential confounders. Candidate covariates for the multivariate models included known correlates of adverse outcomes and covariates differing at baseline between abciximab and eptifibatide groups. Given previous associations with adverse outcomes in the ACUITY data set and other ACS populations, covariates that were forced into the final models were abciximab versus eptifibatide (main effect), age, diabetes, renal insufficiency, baseline cardiac biomarker elevation, ST-segment deviation ≥1 mm, Thrombolysis In Myocardial Infarction (TIMI) risk score, study antithrombin, and treatment with a thienopyridine. A propensity score (including region of enrollment as a covariate) was derived to predict the probability of treatment with abciximab versus eptifibatide using a logistic regression model, and this was forced into the model as well. Additional covariates considered for model selection (stepwise selection with entry criterion of p <0.10 and retention at p <0.05) because of differences between treatment groups were gender, weight, hypertension, hyperlipidemia, previous myocardial infarction, previous CABG, previous PCI, duration from randomization to study antithrombin, antithrombin medications before randomization, and duration from study antithrombin to actual PCI.
Outcomes are presented as crude (unadjusted) odds, propensity-adjusted odds (adjustment only by propensity score), covariate-adjusted odds (adjustment for confounders including propensity score), and covariate-adjusted odds with stratification by the propensity score, which was considered the most robust model. In the final model, region of enrollment was removed from the propensity score and included as a separate covariate in the model to avoid colinearity. First-order interaction terms of the main effect (abciximab vs eptifibatide) were tested by study antithrombin (any heparin vs bivalirudin, unfractionated heparin vs enoxaparin) as well by as region of enrollment.
Results
The study cohort consisted of 2,211 patients, including 835 (37.8%) treated with abciximab and 1,376 (62.2%) treated with eptifibatide ( Figure 1 ). The baseline characteristics of the abciximab- and eptifibatide-treated groups are listed in Table 1 . Compared to patients treated with eptifibatide, those treated with abciximab were older but had fewer cardiovascular risk factors and less frequently had previous myocardial infarctions or had undergone coronary revascularization in the past. However, abciximab-treated patients were more likely to have positive cardiac biomarkers and ST-segment deviation on presentation. More low-risk patients were treated with abciximab, and more intermediate-risk patients were treated with eptifibatide; TIMI high-risk patients were equally likely to be treated with each agent. Patients treated with abciximab had slightly shorter times from hospital admission to first study randomization but slightly longer times from randomization to PCI (and from study antithrombin to PCI; Table 2 ). Patients treated with abciximab had a similar proportion of treatment with bivalirudin but had a greater proportion of treatment with enoxaparin (29.8% vs 20.9% for eptifibatide, p <0.001) and correspondingly lower use of unfractionated heparin. Additionally, thienopyridines were administered before PCI more frequently in abciximab-treated patients (76.3% vs 61.0% for eptifibatide, p <0.001). Per protocol, the total duration of GP IIb/IIIa infusion was greater in eptifibatide-treated patients ( Table 2 ).
| Variable | Abciximab | Eptifibatide | |
|---|---|---|---|
| (n = 835) | (n = 1,376) | p Value | |
| Age | 64 (55–73) | 61 (53–71) | <0.001 |
| Men | 75.4% (630/835) | 71.7% (986/1,376) | 0.051 |
| Diabetes mellitus | 24.6% (203/825) | 28.3% (388/1,370) | 0.06 |
| Insulin-requiring diabetes | 8.4% (69/825) | 7.7% (106/1,370) | 0.60 |
| Hypertension (on medication) | 59.5% (493/829) | 69.2% (946/1,367) | <0.001 |
| Hyperlipidemia (on medication) | 46.9% (381/813) | 60.4% (821/1,359) | <0.001 |
| Current smokers | 32.5% (263/808) | 32.8% (447/1,363) | 0.91 |
| Previous myocardial infarction | 23.1% (189/818) | 34.5% (461/1,337) | <0.001 |
| Previous coronary angioplasty | 30.2% (250/828) | 44.0% (603/1,370) | <0.001 |
| Previous coronary bypass surgery | 10.8% (90/832) | 20.2% (277/1,374) | <0.001 |
| Previous cerebrovascular event | 8.7% (73/835) | 7.7% (106/1,376) | 0.39 |
| Renal insufficiency | 19.8% (158/797) | 16.8% (217/1,294) | 0.08 |
| Baseline cardiac biomarker elevation | 71.6% (561/784) | 59.3% (764/1,288) | <0.001 |
| ST-segment deviation ≥1 mm | 46.6% (389/835) | 27.5% (379/1,376) | <0.001 |
| Baseline cardiac biomarker elevation or ST deviation | 85.2% (689/809) | 69.1% (906/1,311) | <0.001 |
| TIMI risk score | |||
| Low (0–2) | 19.9% (160/805) | 14.7% (169/1,150) | 0.003 |
| Intermediate (3–4) | 48.2% (388/805) | 56.4% (649/1,150) | <0.001 |
| High (5–7) | 31.9% (257/805) | 28.9% (332/1,150) | 0.15 |
| Variable | Abciximab | Eptifibatide | p Value |
|---|---|---|---|
| (n = 835) | (n = 1,376) | ||
| Time from admission to first randomization (hours) | 5.40 (1.78–14.63) | 6.86 (1.89–16.40) | 0.04 |
| Duration from randomization to study antithrombin (hours) | 0.37 (0.17–0.85) | 0.58 (0.27–1.13) | <0.001 |
| Time from randomization to PCI (hours) | 5.08 (1.88–22.80) | 4.70 (2.00–18.78) | 0.014 |
| Time from study antithrombin to PCI (hours) | 4.26 (1.33–21.47) | 3.75 (1.25–16.53) | <0.001 |
| Antithrombin medications (after randomization) | |||
| Bivalirudin | 47.3% (395/835) | 49.6% (682/1,376) | 0.30 |
| Unfractionated heparin | 21.8% (182/835) | 27.0% (371/1,376) | 0.007 |
| Enoxaparin | 29.8% (249/835) | 20.9% (288/1,376) | <0.001 |
| Aspirin use or administration before PCI | 98.0% (818/835) | 97.9% (1,347/1,376) | 0.91 |
| Thienopyridine use or administration before PCI | 76.3% (637/835) | 61.0% (836/1,370) | <0.001 |
| Total duration of GP IIb/IIIa infusion (hours) | 12.24 (11.98–12.97) | 17.00 (12.13–18.18) | <0.001 |
GP IIb/IIIa inhibitor choice was also highly associated with geographic region of enrollment ( Table 3 ). The preference for abciximab or eptifibatide varied substantially among countries and was highly skewed within a given country, with only 3 countries (Sweden, Denmark, and Norway) using <75% of the preferred drug (in other words, in only 3 countries was the less commonly used agent used >25% of the time). The proportion of abciximab use (among all abciximab use) that occurred within North American versus non–North American countries was 16.9% versus 83.1% (p <0.001), whereas the frequency of eptifibatide use within the 2 regions was 87.4% versus 12.6%, respectively (p <0.001).
| Country | Abciximab | Eptifibatide |
|---|---|---|
| United States | 9.9% (126/1,273) | 90.1% (1,147/1,273) |
| Canada | 21.4% (15/70) | 78.6% (55/70) |
| Total, North America | 10.5% (141/1,343) | 89.5% (1,202/1,343) |
| Germany | 75.7% (299/395) | 24.3% (96/395) |
| Spain | 100.0% (84/84) | 0.0% (0/84) |
| Australia | 100.0% (49/49) | 0.0% (0/49) |
| Austria | 93.2% (55/59) | 6.8% (4/59) |
| Italy | 86.5% (32/37) | 13.5% (5/37) |
| New Zealand | 3.4% (1/29) | 96.6% (28/29) |
| Belgium | 100.0% (35/35) | 0.0% (0/35) |
| Sweden | 74.4% (32/43) | 25.6% (11/43) |
| United Kingdom | 100.0% (27/27) | 0.0% (0/27) |
| France | 75.8% (25/33) | 24.2% (8/33) |
| Denmark | 71.0% (22/31) | 29.0% (9/31) |
| Netherlands | 100.0% (21/21) | 0.0% (0/21) |
| Norway | 53.3% (8/15) | 46.7% (7/15) |
| Finland | 14.3% (1/7) | 85.7% (6/7) |
| Poland | 100.0% (3/3) | 0.0% (0/3) |
| Total, non–North America | 80.0% (694/868) | 20.0% (174/868) |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


