Management of postcatheterization femoral artery pseudoaneurysm (FAP) is problematic. Ultrasound-guided compression (UGC) is painful and cumbersome. Thrombin injection is costly and may cause thromboembolism. Ultrasound-guided para-aneurysmal saline injection (PASI) has been described but was never compared against other treatment methods of FAP. We aimed at comparing the success rate and complications of PASI versus UGC. We randomly assigned 80 patients with postcatheterization FAPs to either UGC (40 patients) or PASI (40 patients). We compared the 2 procedures regarding successful obliteration of the FAP, incidence of vasovagal attacks, procedure time, discontinuation of antiplatelet and/or anticoagulants, and the Doppler waveform in the ipsilateral pedal arteries at the end of the procedure. There was no significant difference between patients in both groups regarding clinical and vascular duplex data. The mean durations of UGC and PASI procedures were 58.14 ± 28.45 and 30.33 ± 8.56 minutes, respectively (p = 0.045). Vasovagal attacks were reported in 10 (25%) and 2 patients (5%) treated with UGC and PASI, respectively (p = 0.05). All patients in both groups had triphasic Doppler waveform in the infrapopliteal arteries before and after the procedure. The primary and final success rates were 75%, 92.5%, 87.5%, and 95% for UGC and PASI, respectively (p = 0.43). In successfully treated patients, there was no reperfusion of the FAP in the follow-up studies (days 1 and 7) in both groups. In conclusion, ultrasound-guided PASI is an effective method for the treatment of FAP. Compared with UGC, PASI is faster, less likely to cause vasovagal reactions, and can be more convenient to patients and physicians.
Femoral artery pseudoaneurysm (FAP) is a troublesome groin complication related to the femoral arterial access. The incidence of FAP may reach up to 6% and is more likely with complex structural, coronary, or peripheral interventions, using large-sized introducer sheath and potent antithrombotic therapy. FAP may cause compression neuropathy, venous thrombosis, critical limb ischemia, skin necrosis, infection, or may even rupture. Small asymptomatic pseudoaneurysms can be managed conservatively unless they are still present on a follow-up duplex scan 2 months later. In contrast, large (>2 cm), symptomatic, or complicated FAPs should be treated. The most commonly used techniques are ultrasound-guided compression (UGC), surgical repair, and thrombin injection. UGC is safe and effective but entails a lengthy procedure, patient discomfort, and a high failure rate in patients receiving dual antiplatelet and/or anticoagulant therapy. With surgical repair, the incidence of wound-healing disorders and permanent neuralgias is 32% and lymphatic leakage is 40%. Ultrasound-guided percutaneous thrombin injection is a costly procedure, and serious complications including thromboembolism and allergic reaction secondary to thrombin injection have been described. Another, minimally invasive, technique has been described by Gehling et al and uses ultrasound-guided para-aneurysmal saline injection (PASI) for treating FAPs. This technique has not been compared systemically with UGC or thrombin injection. We aimed at comparing the success rate and complications of PASI versus UGC.
Methods
From December 2009 till December 2012, we recruited 80 patients with post–cardiac catheterization FAPs. Patients were clinically examined, and the diagnosis of FAP was established after detailed duplex examination, using Advanced Technology Laboratories HDI 5,000, Siemens Elegra (Siemens Medical Solutions USA, Inc., Mountain View, California), and HP Sonos 2,000 systems (Philips Koninklijke, Eindhoven). All had high-resolution broadband width linear array transducers L5-7/10 MHz ( Figures 1 and 2 ). All included patients had either large pseudoaneurysms >2 cm and/or symptoms related to the pseudoaneurysm. Exclusion criteria were patient refusal to grant consent or to be randomized, presence of synthetic arterial graft, rapidly expanding pseudoaneurysm, skin ischemia or compromised soft tissue viability, infection at the anticipated site of compression or infected pseudoaneurysm, presence of uncontrollable hemorrhage, and limb-threatening ischemia resulting from a pseudoaneurysm. The study was approved by our local ethical committee. Informed consent was obtained from all patients after being instructed about the nature and risks of the study and both procedures.
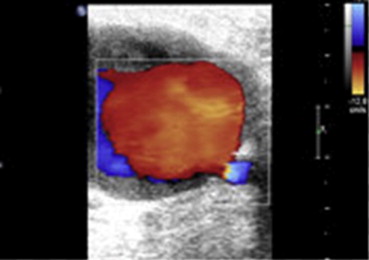
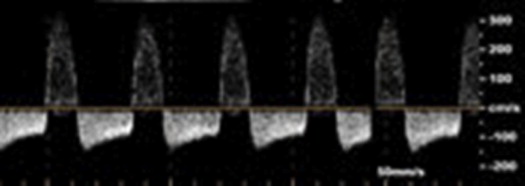
The following data were reported for all included patients: age, gender, presence of diabetes mellitus, hypertension, obesity defined as body mass index ≥30 kg/m 2 , onset of presentation after catheterization: within 3 days, 3 to 7 days, or >7 days, type of the catheterization done, gauge of introducer sheath, and the use of single (aspirin or clopidogrel) or dual antiplatelet (aspirin plus clopidogrel) and/or anticoagulant therapy, with measurement of the international normalized ratio value for patients receiving warfarin. Discontinuation of antiplatelet and/or anticoagulation was either left to the discretion of the referring physician or performed after failed attempt of either UGC or PASI.
The following parameters were reported during duplex scanning:
- 1.
The Doppler waveform in the ipsilateral infrapopliteal arteries (triphasic, monophasic damped, or absent).
- 2.
The presence or absence of spontaneous thrombosis within the FAP cavity or lining its wall.
- 3.
Number of lobes of FAP, either single lobe (unilocular) or multilocular.
- 4.
Feeding artery: common femoral, superficial femoral, or profunda femoral artery.
- 5.
Number of necks communicating the feeding artery with the FAP (single or multiple).
- 6.
Width and length of the neck, if there were multiple necks, only the measurements of the largest one was reported.
- 7.
FAP length, width, height, and volume. The volume was calculated by the following formula:
( Length × width × height × 0.5 )
.
In cases with multiloculated FAP, the volume of the proximal lobe only was calculated.
The 80 patients who had definitive diagnosis of FAP were randomly assigned to treatment by either UGC or PASI. UGC was performed after the feeding artery and the neck of the aneurysm were properly identified by ultrasound. Compression was then done with the linear array ultrasound transducer aiming to obliterate the flow within the neck and FAP completely. Pressure was applied in cycles of 10-minutes compression alternating with 1 to 2 minutes without compression to re-evaluate perfusion of the FAP, start of thrombosis, reposition the probe at the proper site and give some relief for the patient.
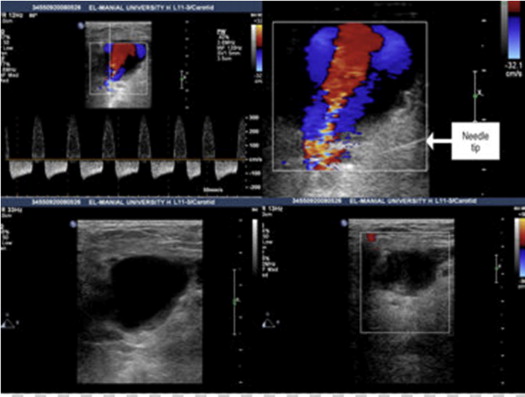
PASI was done by the following technique: after the patient’s skin at the groin was prepared with povidone iodine, sterile drapes were placed around the inguinal area. The ultrasound transducer was covered with a sterile laminated bag, and sterile gel was applied to the patient’s skin to prevent infection. All patients had a peripheral intravenous line for atropine injection if necessary. After administration of subcutaneous anesthesia with 10 ml of 2% lidocaine, an 18-gauge needle mounted on a plastic syringe filled with 0.9% saline solution (drawn from a 500-ml saline bag) was advanced and positioned within 2 to 5 mm from the neck communicating the femoral artery and the FAP. After confirmation of extravascular and extra-aneurysmal needle position, saline solution was slowly injected until the resultant tissue swelling completely obliterated the FAP neck ( Figure 3 ). Puncture of the femoral artery and/or the FAP was avoided in all patients. Repositioning of the needle and reinjection on the opposite side of the neck was performed if ultrasound monitoring indicated insufficient compression of the pseudoaneurysm neck. After saline injection, manual compression was applied for a 5-minute period.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


