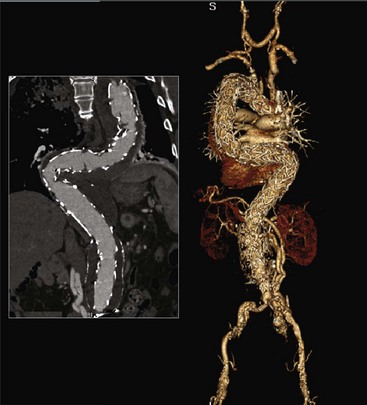
Chapter 36 Combined Endovascular and Surgical (Hybrid) Approach to Aortic Arch and Thoracoabdominal Aortic Pathology
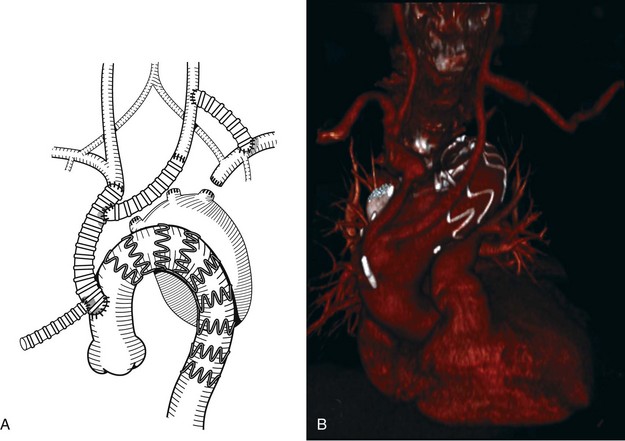
A combined approach consisting of surgical bypass and aortic endovascular stent graft placement was first used at the University of California–Los Angeles to treat a type IV thoracoabdominal aneurysm in 1999. This procedure was performed to minimize morbidity and mortality of an open repair in a high-risk patient.1 As this technique has evolved, it is now used to treat more extensive aneurysms of the aortic arch and thoracoabdominal aorta (Figure 36-1, see color plate).
A hybrid repair of thoracoabdominal aneurysms avoids proximal aortic cross clamping and thoracotomy, and it minimizes the visceral ischemia time. For the aortic arch, avoidance of hypothermic circulatory arrest makes a hybrid approach appealing. The use of thoracic stent-grafts in association with open surgical debranching is considered an off-label application of the device.2
Patient Selection
Open versus Hybrid Approach
Aortic Arch Aneurysm
Open repair of aortic arch aneurysms require a sternotomy, extracorporeal circulatory support often with deep hypothermic circulatory arrest, and hemiarch or total arch replacement.3 In cases of concomitant descending thoracic aorta involvement, an “elephant trunk” technique can be used where a second intervention addresses the descending thoracic aorta component.4,5 In high-risk patients, a hybrid approach is attractive because it avoids the need for hypothermic arrest, and in cases of descending thoracic involvement the procedure can be done in one stage.
Safi and colleagues6 published one of the largest case series of open aortic arch aneurysm repair with thoracic or thoracoabdominal aortic involvement in 2004. They replaced the ascending aorta and aortic arch, leaving an elephant trunk in the descending thoracic aorta in 218 patients. Strokes occurred in 2.7%, and 30-day mortality was 8.7%. Of the surviving 199 patients, 2% expired before returning for the second-stage thoracic or thoracoabdominal aortic repair completion. Of those completing the second surgery, 9.7% died within 30 days of the second surgery.6 Smaller case series of open arch repair in association with ascending or thoracoabdominal aortic aneurysms involve heterogeneous populations and report a wide range of perioperative mortality from 5.3% to 30.8%.3
There have been no randomized trials of hybrid versus open repair, but institutions have compared cohorts of open and hybrid repairs. At the University of Florida in Gainesville, they identified 58 patients with aortic arch aneurysms and descending thoracic aortic involvement. An open cohort using a two-staged approach with aortic arch replacement and elephant trunk repair (n = 21) was compared to a cohort using a hybrid approach (n = 37). Rates of spinal cord ischemia (0% vs. 0%), stroke (10% vs. 11%), 30-day mortality (19% vs. 16%), and survival at 12 months (73% vs. 72%) were similar between the open and hybrid cases. These comparable results were present even though 68% of the hybrid cases still used cardiopulmonary bypass.7
In 2010, a more recent, single institution study compared 27 hybrid arch procedures with a contemporary series of 45 cases of open arch repairs. The incidence of permanent cerebral neurologic deficit (4% vs. 9%) and in-hospital mortality (11% vs. 16%) were similar for the hybrid versus open cohort. In the open arch group, it was noted that patients older than 75 years had significantly higher mortality than those younger than 75 years (36% vs. 9%; p = 0.05).8
Two metaanalyses of hybrid case series have been published. Antoniou and colleagues9 reviewed 18 studies with a total of 195 patients. Complete arch debranching was performed in 122 patients (63%). The overall technical success rate was 86%. Overall perioperative morbidity and mortality rates were 21% and 9%, respectively. The stroke rate was 7%, and four aneurysm-related deaths were reported during follow-up (2%). No long-term data was reported.9 A second metaanalysis identified 15 studies with 463 patients in studies published up to May 2008. The overall 30-day mortality was 8.3%, stroke rate was 4.4%, paraplegia rate was 3.9%, and the endoleak rate was 9.2%. Treatment on-pump or off-pump did not affect any of the endpoints.10
Thoracoabdominal Aneurysm
Open repair of thoracoabdominal aneurysms involve a laparotomy, thoracotomy, proximal aortic cross clamp, and global visceral ischemia. Techniques to optimize open repairs have included left heart bypass and retrograde perfusion of the renal and mesenteric vessels. In high-volume centers, case series of open thoracoabdominal aneurysm repair demonstrate reasonable mortality of 5% to 8.3% with rates of spinal cord ischemia from 3.8 to 16%.11–14 Late survival at 5, 10, and 15 years were 54% to 67%, 29%, and 21%, respectively.13,14
In a contemporary series of open thoracoabdominal aneurysm repair, 305 patients were reviewed.15 Twenty percent underwent an urgent or emergent repair, and 57% were Crawford extents type I or II. The majority had cerebrospinal fluid drainage (97%) and left heart bypass (89%). Operative mortality was 8%, renal failure necessitating hemodialysis at discharge was 6%, and permanent paraplegia was 3%. Actuarial survival at 2 years was 79%.15
Despite these single center outcomes, database outcomes find mortalities to be more dismal. In California, after open repair, patient mortality was 19% at 30 days and 31% at 1 year. An increase in 1-year mortality was noted for patients of increasing age: 18% for patients 50 to 59 years old, versus 40% for patients 80 to 89 years old.16 Nationwide, in-hospital mortality was found to be 22.3%. Higher mortality was seen when comparing low- versus high-volume hospitals (27.4% vs. 15.0%; p < 0.001) and low- versus high-volume surgeons (25.6% vs. 11.0%; p < 0.001).17
There have been no randomized comparisons of open versus hybrid thoracoabdominal aneurysm repair. At the Massachusetts General Hospital they compared a contemporary cohort of open (n = 77) versus hybrid (n = 23) thoracoabdominal repairs. Of note, the hybrid patients were not open surgical candidates. Although not significant, the hybrid groups had a higher 30-day mortality (17.4% vs. 7.8%; p = 0.23), in-hospital mortality (26.1% vs. 10.4%; p = 0.27), and reoperation rates (39.1% vs. 20.8%; p = 0.03). However, paraplegia rates (hybrid, 4.3%, vs. open, 3.9%; p = 0.98) and 1-year survival (hybrid, 68%, vs. open, 73%) were similar for both groups.18 Additional series of hybrid repairs are limited to small, heterogeneous patient populations, with varying extents of type II and III thoracoabdominal aneurysms.
Debranching the Aortic Arch
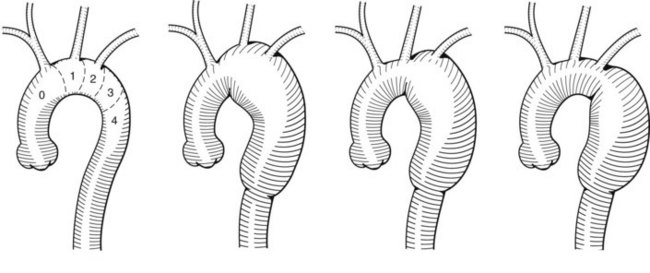
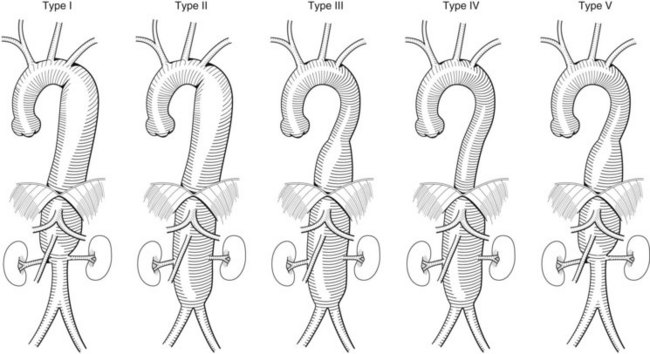
The surgical component of a hybrid repair to the aortic arch is used to create a proximal seal zone for an endograft. This is done to treat the proximal extent of thoracic or thoracoabdominal aortic aneurysms or to treat isolated aortic arch aneurysms. The anatomy of the aneurysm will determine which vessels need to be addressed. A classification scheme separates the aortic arch into proximal seal zones and identifies which vessels will be affected19 (Figure 36-2). Zone 0 involves the origin of the innominate artery, and a stent-graft landing there compromises blood flow to the left subclavian, left common carotid, and innominate arteries. Zone 1 involves the orifice of the left common carotid artery, and a stent-graft landing there compromises the left subclavian and left common carotid circulation. Zone 2 involves the origin of the left subclavian artery, and a stent-graft landing there affects only the left subclavian artery. Zones 3 and 4 are both distal to the left subclavian artery, within the descending thoracic aorta, leaving the arch vessels unaffected.
Left Subclavian Artery
Early in the endovascular treatment of thoracic aneurysms, coverage of the left subclavian artery (zone 2) was routinely done and appeared safe. It was assumed a patent right vertebral artery would provide enough collateral circulation to both the basilar system and the left subclavian artery via retrograde flow from the left vertebral artery.20,21 More recent data are conflicting. Metaanalysis suggest no neurologic benefit from subclavian revascularization,22 but in a registry from Europe, multivariate regression analysis found the risk of spinal cord ischemia to increase with left subclavian artery coverage.23 Late development of subclavian steal and arm claudication was seen in 15% of patients in one series requiring further intervention.24 We recommend revascularization of the left subclavian artery in all elective cases. In unstable patients, coverage of the left subclavian artery may be reasonable.
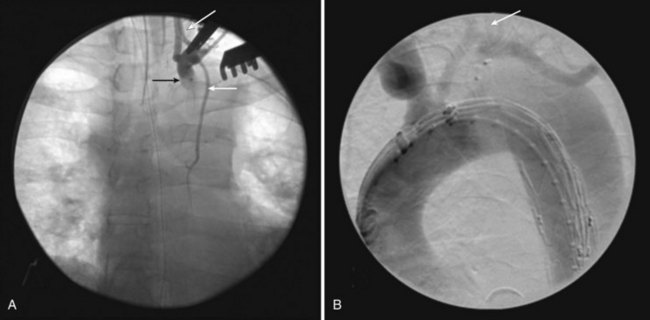
A left supraclavicular incision is used to perform either a left subclavian to carotid artery transposition or left common carotid to subclavian artery bypass. Regardless of the method, left vertebral artery flow should be preserved. In all cases where a bypass is used, ligation or endovascular occlusion of the subclavian proximal to the vertebral origin is recommended to avoid a type II endoleak. Endovascular occlusion can be done using an Amplatzer plug (ADA Medical Corporation, Plymouth, Minn.) or iliac occlusion plug (Zenith Iliac Plug, Cook, Bloomington, IN or Talent Occluder, Medtronic, Minneapolis, MN) deployed in retrograde fashion though the distal anastomosis of the carotid-subclavian artery bypass (Figure 36-3). If an Amplatzer plug is used, it should be done after the aortic stent graft is deployed to prevent developing thrombus from embolizing while there is still antegrade flow through the device and subclavian artery. In cases where a left internal mammary artery has been used for a coronary bypass graft, a transposition cannot be performed. In constructing the carotid-subclavian artery bypass, the subclavian anastomosis should be distal to the origin of the internal mammary artery, thereby maintaining native flow during sewing of the anastomosis. After the anastomosis and bypass are complete, the origin of the left subclavian artery is ligated, and retrograde flow will fill the internal mammary artery.25
Partial Arch Debranching
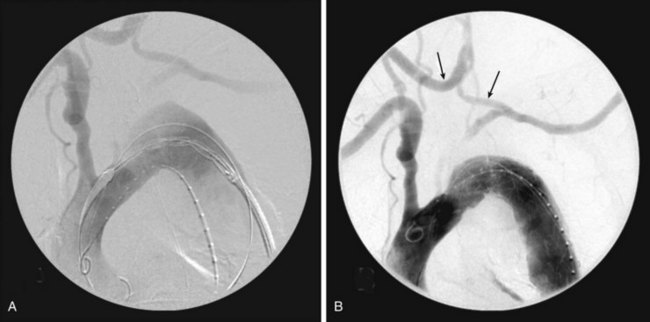
Partial arch debranching involves revascularization of the left common carotid and left subclavian arteries using the right common carotid artery as inflow, in preparation to deploy an endograft just distal to the innominate artery origin (zone 1). This can be done with extraanatomic bypasses, without the need for a sternotomy or thoracotomy (Figure 36-4).
The left subclavian artery revascularization is performed as mentioned previously. This is followed by a right carotid to left carotid artery bypass through bilateral neck incisions made anterior to the sternocleidomastoid muscle to expose the common carotid arteries. A retropharyngeal or subcutaneous tunnel is created between the two common carotid arteries, and a bypass is performed with a Dacron graft. The tunnel is made with a gentle downward curve. If placed subcutaneously, carrying the tunnel behind the manubrium gives the best cosmetic result. Damage to this bypass can occur if a sternotomy is ever needed, and patients should be so informed.26 The left common carotid artery is ligated proximal to the distal carotid-carotid artery bypass anastomosis. Care is taken to avoid bilateral recurrent laryngeal or vagus nerve injury.
Complete Arch Debranching
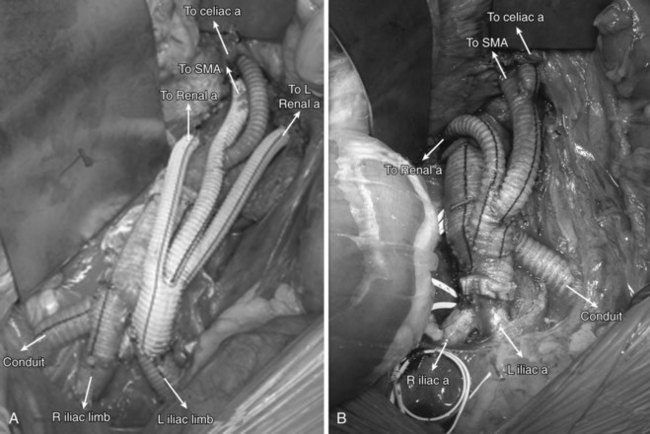
Complete aortic arch debranching involves revascularization of the innominate, left carotid and left subclavian arteries to create a landing zone in the ascending aorta proximal to the innominate artery (zone 0). This is most often done through a median sternotomy27 and cardiopulmonary bypass is typically not necessary. A side biting clamp is placed on the ascending aorta and either a small bifurcated Dacron graft or a larger tube graft (8 to 12 mm) with a side arm (6 to 8 mm) is fashioned to bypass the right innominate and left common carotid arteries, both in an end to end fashion (Figures 36-5 [see color plate] and 36-6). The latter option is easier to configure and avoids compression or kinking after sternotomy closure.
The tortuosity and distance from the femoral artery to proximal aortic arch can make tracking an endovascular device difficult. In addition, the endograft delivery system may not be long enough to reach the ascending aorta. The addition of a 10-mm Dacron graft onto the hood of the ascending aortic graft can serve as a conduit for antegrade deployment of the stent graft28 (see Figures 36-5 and 36-6). It is critical that the location of the conduit to graft anastomosis lead directly into the aorta. Tension at the heel of the ascending aortic graft anastomosis can develop during deployment because of the angle of entry of the deployment catheter; this risks a tear of the aorta and is to be avoided. Tunneling the conduit through an appropriately located stab wound can reduce this risk.29
Frozen Elephant Trunk
When aneurysms involve the ascending aorta, there is no proximal landing zone for a stent graft. To reconstruct these aneurysms, the frozen elephant trunk is a one-stage hybrid technique. Hypothermic cardiac arrest is required, hence the term frozen. The ascending aorta is replaced with a Dacron graft. A bypass is then taken off this graft to the innominate and left carotid arteries. The arch is then opened, and a modified Dacron graft with a stent attached distally is constrained in a sheath and deployed under fluoroscopy into a distal landing zone, usually the descending thoracic aorta. The proximal end of the modified Dacron graft is then sewn to the ascending aortic graft completing the reconstruction. This technique eliminates the two-stage operation needed with standard aortic arch and elephant trunk operations, but it requires circulatory arrest and for this reason will not be discussed further in this chapter.30–33
Debranching Thoracoabdominal Aneurysms
Given that the visceral vessels are in close proximity to one another, all four vessels (celiac, SMA, and bilateral renal arteries) will need to be bypassed in most hybrid thoracoabdominal aneurysm repairs. In pararenal aneurysms, debranching one or both kidneys may provide the needed seal zone for a hybrid repair.34 In patients with preexisting renal failure, on hemodialysis, only the mesenteric arteries require debranching.
Origin of the Visceral Bypass Grafts
Occasionally patients will have had a prior aortic repair, and this preexisting graft can serve as a takeoff for a retrograde visceral graft. If not, the anatomy of the aneurysm will dictate where a bypass graft can originate. The Crawford classification of thoracoabdominal aneurysms can help predict this (Figure 36-7). It is preferable not to use the external iliac arteries given their small size and presence of atherosclerosis, which may not properly support circulation to the entire viscera.
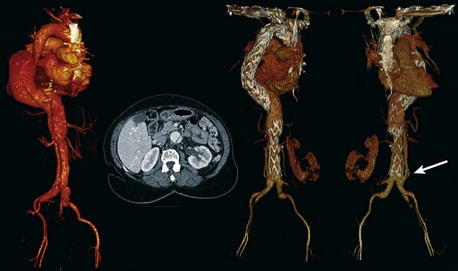
In a type I Crawford thoracoabdominal aneurysm, the native common iliac artery or infrarenal aorta is adequate for use as a graft origin (Figure 36-8, see color plate). In a type II and III Crawford thoracoabdominal aneurysm, aneurysmal involvement of the infrarenal aorta and common iliac arteries makes this a more challenging hybrid repair. If the aneurysm is continuous throughout the iliacs, without a spared segment of nonaneurysmal aorta, a hybrid repair is not feasible and an open repair is the only option (Figure 36-9, see color plate). If there is a region of nonaneurysmal aorta that can be clamped, a tube or bifurcated aortoiliac graft can be placed for a distal landing zone, and the debranching graft sewn onto the aortic graft (Figure 36-10).
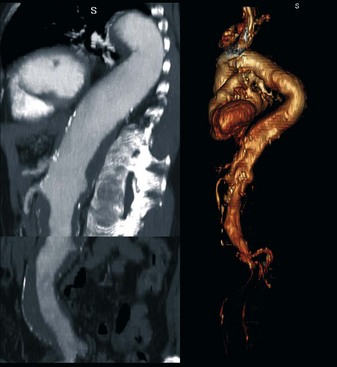
FIGURE 36-9 Type II thoracoabdominal aneurysm. Entire aorta is aneurysmal without a spared segment for a hybrid repair.
Abdominal Debranching techniques
Patients are placed supine and a midline transabdominal incision is preferred. A retroperitoneal approach can be used, but access to the right renal artery can be difficult. To create a retrograde debranching bypass graft, a trifurcated Dacron prosthesis is preferred. Two limbs are used to bypass the right and left renal arteries with an end-to-end distal anastomosis if feasible. The third limb is used for the SMA in an end-to-side fashion. A jump graft is then taken off the SMA graft, to the celiac artery. The routing of the retrograde bypass grafts will be determined by whether the grafts originate from the right or left aortoiliac systems (Figure 36-11).
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree