, Brian L. Edlow1, David M. Greer2, David M. Greer3 and David M. Greer4, 5
(1)
Harvard Medical School Department of Neurology, Massachusetts General Hospital, Boston, MA, USA
(2)
Yale University School of Medicine, New Haven, CT, USA
(3)
Department of Neurology, New Haven, CT, USA
(4)
Neurology Residency Program, New Haven, CT, USA
(5)
Neurosciences Intensive Care Unit, Department of Neurology, Yale-New Haven Hospital, New Haven, CT, USA
Abstract
Approximately 450,000 Americans suffer cardiac arrest annually. Coma after successful emergency resuscitation from circulatory arrest, as a result of anoxic-ischemic brain damage, presents challenges for neurological management and prognostication. Therapeutic hypothermia (TH) and aggressive post-resuscitation medical management have significantly improved neurological outcomes in cardiac arrest patients over the past decade. At the same time, neurological prognostication, traditionally based on clinical signs and auxiliary tests validated before widespread use of TH, has become more difficult, with accumulating evidence that TH alters the ability of these indicators to reliably predict poor neurological outcomes. Optimal outcomes and avoidance of “self-fulfilling” prophecies (predictions of poor prognosis leading to premature decisions to withdraw life-sustaining therapy) requires skillful management of the comatose postanoxic patient and an integrative, multimodal approach to neurological prognostication.
Abbreviations
ACLS
Advanced cardiac life support
BP
Blood pressure
cEEG
Continuous electroencephalography
CPR
Cardiopulmonary resuscitation
DPD
Delayed posthypoxic demyelination
ECS
Electrocerebral silence
EEG
Electroencephalography
EMS
Emergency medical services
ESE
Electrographic/subtle status epilepticus
FPR
False positive rate
GPEDs
Generalized periodic epileptiform discharges
ICP
Intracranial pressure
LAS
Lance adams syndrome
MSE
Myoclonic status epilepticus
NSE
Neuron-specific enolase
PEA
Pulseless electrical activity
PED
Periodic epileptiform discharges
PMSE
Postanoxic myoclonic status epilepticus
PSE
Postanoxic status epilepticus
PVS
Persistent vegetative state
ROSC
Return of spontaneous circulation
SPECT
Single-photon emission computed tomography
SSEP
Somatosensory evoked potentials
TCD
Transcranial doppler ultrasound
TH
Therapeutic hypothermia
VF
Ventricular fibrillation
VT
Ventricular tachycardia
Introduction
Approximately 450,000 Americans suffer cardiac arrest annually. Coma after successful emergency resuscitation from circulatory arrest, as a result of anoxic-ischemic brain damage, presents challenges for neurological management and prognostication. Therapeutic hypothermia (TH) and aggressive post-resuscitation medical management have significantly improved neurological outcomes in cardiac arrest patients over the past decade. At the same time, neurological prognostication, traditionally based on clinical signs and auxiliary tests validated before widespread use of TH, has become more difficult, with accumulating evidence that TH alters the ability of these indicators to reliably predict poor neurological outcomes. Optimal outcomes and avoidance of “self-fulfilling” prophecies (predictions of poor prognosis leading to premature decisions to withdraw life-sustaining therapy) requires skillful management of the comatose postanoxic patient and an integrative, multimodal approach to neurological prognostication.
Epidemiology and Pathophysiology of Postanoxic Coma
<10 % survival for out-of-hospital cardiac arrest after cardiopulmonary resuscitation (CPR) [1]
<20 % survival to discharge for in-hospital cardiac arrest after CPR [2]
Duration of anoxia prior to CPR and duration of CPR correlate with poor outcome (Fig. 28-1) [4] (These durations are not key variables in prognostication algorithms, however.)
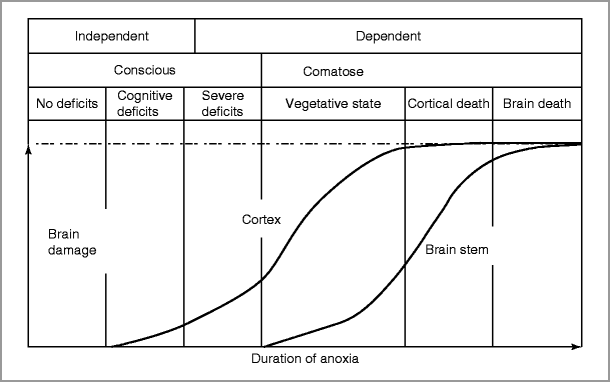
Figure 28-1
Schematic relationship between duration of anoxia and degree of brain damage and neurological outcomes (Adapted from Khot and Tirschwell [3])
Brain Death (Table 28-1)
Table 28-1
Clinical criteria for brain death
Clinical measure | Findings consistent with brain death |
|---|---|
Coma | No eye opening or responsiveness, other than spinally-mediated |
Not cerebrally-mediated movement to noxious stimuli with supraorbital pressure and deep nail bed pressure in all 4 extremities | |
Absence of brain Stem reflexes | Pupils: |
Fixed pupils, even with bright light and magnifying glass | |
Ocular movements: | |
No oculocephalic reflex. Only test if C-spine integrity has been ensured | |
No oculovestibular reflex (absent caloric stimulation response). Confirm integrity of tympanic membrane and absence of significant blood/wax in external auditory canal, elevate head-of-bed to 30˚ and irrigate external auditory canal with 30–50 mL of ice-water. Observe for ocular response (1 min) then repeat on contralateral side after at least 5 min delay | |
Facial motor responses: | |
No corneal reflex to touch with cotton swab | |
No facial grimace to deep pressure on nailbeds, supraorbital ridge, or temporomandibular joint | |
Pharyngeal and tracheal reflexes: | |
No gag with stimulation of posterior pharynx | |
No cough to bronchial suctioning | |
Apnea testing | Prerequisites and preparation: |
Core temp ≥36.5 °C (96.8 °F) | |
SBP > 100: If pt requiring high doses of pressors or experiencing significant cardiac arrhythmias, consider ancillary testing instead of proceeding with apnea testing | |
Euvolemia: If diabetes insipidus present, need + fluid balance over prior 6 h | |
Adjust ventilator settings to achieve arterial pH 7.35–7.45 and PCO2 35–45 mmHg ≥ 20 min prior to apnea testing (or to patient’s baseline, if known CO2 retainer) | |
Pre-oxygenate with 100 % fiO2 for at least 5 min to PaO2 >200 mmHg | |
Procedure: | |
Disconnect pt from ventilator | |
Administer 100 % O2 at 8–10 L/min via endotracheal tube or tracheostomy to level of carina immediately after disconnecting vent | |
Observe for respiratory movements for approx 8 min | |
After 8 min period elapses, check ABG to measure O2, PCO2, and pH | |
Reconnect pt to ventilator after ABG is drawn | |
If during 8 min period off of ventilator patient develops cyanosis, SBP <90 mmHg, O2 desaturation <85 % for >30 seconds, or hemodynamically significant cardiac arrhythmias, then discontinue apnea testing, draw STAT ABG and reconnect ventilator and hyperventilated the patient briefly to correct acidosis | |
Positive apnea test ( consistent with brain death ): | |
No respiratory movements | |
ABG Criteria: PCO2 ≥60 mmHg or PCO2 increase ≥ 20 mmHg from baseline in known CO2 retainers | |
Apnea test considered positive if stopped early as long as no respiratory movements are observed and ABG criteria are met | |
Negative apnea test: | |
Respiratory movements observed OR ABG criteria not met after sufficient time elapsed | |
Indeterminate apnea test: | |
No respiratory movements observed but ABG criteria not met. May repeat test for longer time period if pt clinically stable, again after normalizing the PCO2 and hyperoxygenating the patient, or proceed to ancillary testing |
Patients meeting brain death criteria after cardiac arrest are not candidates for therapeutic hypothermia (TH). However, as the American Academy of Neurology guideline on determining brain death in adults states, “because of the deficiencies in the evidence base, clinicians must exercise considerable judgment when applying the criteria in specific circumstances” [5] In particular, it is rarely possible in the acute setting to determine that loss of neurological function is irreversible (e.g. because of sedative effects and insufficient observation time). Thus, brain death assessment must generally be delayed until after TH.
Therapeutic Hypothermia (TH)
A.
Mechanisms of action
Reduces cerebral metabolic rate and oxygen demand
Reduces cerebral edema and intracranial pressure (ICP) by preserving blood brain barrier integrity
Reduces excitotoxic neuronal injury
Minimizes free radical release
Suppresses inflammation
B.
Evidence
Protocols & inclusion/exclusion criteria vary, but mortality and neurological recovery benefits demonstrated by multiple, randomized trials [6, 7].
Data exist only for out-of-hospital, ventricular fibrillation (VF)/ventricular tachycardia (VT) cardiac arrest; no data for pulseless electrical activity (PEA), asystolic arrest, or in-hospital arrest → therapeutic cooling for these types of cardiac arrest may be applied at the discretion of the clinician [8].
TH NOT proven beneficial for coma after isolated respiratory arrest without cardiac arrest
Elevated temperature (hyperthermia) is detrimental (odds ratio for unfavorable outcome >2) for each 1 °C increase in temperature after arrest [9].
C.
Basic principles of TH
Initiate cooling rapidly; cooling must be initiated within 6 h of Return Of Spontaneous Circulation (ROSC).
Multiple methods may be required to meet temperature goal of 32–34 °C (89–93 °F)
Total cooling period is 24 h; begins when cooling is initiated, NOT upon reaching target temperature
Shivering generates heat → neuronal injury by increasing cerebral metabolism; sedation and paralysis may be necessary for duration of cooling to prevent shivering
D.
Preparation for hypothermia:
Laboratory evaluation: Complete metabolic panel, CBC, PT/PTT, fibrinogen, d-dimer
Place arterial line for blood pressure (BP) monitoring
Place temperature monitor for continuous assessment of core temp → bladder temp probe, or pulmonary artery temp probe if oliguric (bladder temp probe requires presence of urine in bladder)
E.
Eligibility and exclusion criteria for TH (Table 28-2)
Table 28-2
Inclusion criteria and contraindications for therapeutic hypothermia after cardiac arrest
Inclusion: |
Comatose (the state of unresponsiveness) |
Time <6 h since cardiac arrest |
Hemodynamically stable without significant pressor requirement after CPR |
Relative contraindications (hypothermia may carry increased risk): |
Major head trauma: rule out intracranial hemorrhage (ICH) by head CT prior to cooling if clinical suspicion for head trauma at time of arrest |
Recent major surgery (within 14 days) |
Systemic infection/sepsis (hypothermia interferes with immune function) |
Other etiology for coma (e.g. drug/EtOH intoxication, pre-existing coma prior to arrest) |
Active bleeding (hypothermia impairs clotting factor activity) |
Not grounds for exclusion: Administration of thrombolytic, anti-platelet, or anticoagulation meds for cardiac condition is NOT a contraindication to hypothermia |
F.
Therapeutic Hypothermia Protocol (Table 28-3)
Table 28-3
Therapeutic hypothermia (protocols may differ by institution)
External cooling with cooling blankets and ice: |
Obtain two cooling blankets and cables (one machine) to “sandwich” the patient; place sheets between blankets and patient to protect skin |
Use additional cooling methods as needed to bring patient to goal temperature |
Pack ice in groin, sides of chest, axillae, and/or side of neck |
Infuse cold (4 º C) normal saline via peripheral or femoral central venous line (but not via a subclavian or IJ central line) (30 cc/kg over 30 min) |
Medicate for shivering with sedating and paralyzing agents (see below) |
Once goal temperature is reached, remove ice bags and maintain temp using cooling blankets |
Avoid packing ice on top of chest ® may impair ventilation |
External cooling with cooling vest devices: |
Set target temperature goal on device |
Medicate for shivering with sedation and paralyzing agents (see below) |
Consider secondary temperature monitor. Record patient temperature on cooling vest device, secondary temperature source, and follow water temperature of the cooling device. Water temperature indicates the work the device must perform to keep patient at target body temp |
Paralysis and sedation |
Paralyze with cisatracurium: 150 mcg/kg bolus, then continuous infusion of 2 mcg/kg/min |
Sedate with propofol: bolus (optional) 0.3–0.5 mg/kg then continuous infusion of 1 mg/kg/h |
OR: |
Midazolam: bolus (optional) 0.05 mg/kg then continuous infusion of 0.125 mg/kg/h. |
Monitoring and supportive therapy during hypothermia: |
No indication for BIS or train-of-four monitoring during TH |
EEG use at clinician’s discretion; consider to detect subclinical seizure activity |
MAP >90 mmHg to maximize cerebral perfusion; potentially additive neuroprotective effects of high perfusion pressure with hypothermia |
MAP goal may be lowered at discretion of clinician, depending on cardiac effects of high afterload or coronary vasoconstriction |
If serious cardiac dysrhythmias, hemodynamic instability or bleeding develops during cooling, stop the cooling process, and actively re-warm the patient |
Osborn waves (positive deflection between QRS complex and ST segment; see Fig. 28-2 below) or bradycardia may develop during cooling. No indication for specific therapy, but this may impair the ability to detect Brugada syndrome |
Check blood cultures at 12 and 24 h after initiation of cooling (TH may mask infection) |
Check electrolytes, CBC, and glucose at 12 and 24 h (TH may cause hypokalemia, esp. during concurrent insulin administration; rewarming may cause hyperkalemia due to K+ efflux from intracellular compartment) |
Hyperglycemia and increases in serum amylase and lipase may occur during cooling |
Goal CO2 35–45 mmHg: analyze all ABGs at pt’s body temperature |
Examine skin for burns q2 h if using cold blankets |
Rewarming: |
Basic principles: |
Do NOT rewarm faster than 0.25 °F/h; passive or controlled rewarming should take 8–12 h |
Shunting of cardiac output to re-opening peripheral vascular beds may cause hypotension |
Monitor closely for hypotension, hyperkalemia |
Aim for normothermia once rewarming phase is completed |
Maintain paralytic and sedative therapy until temperature of 36 °C (96.8 °F) is reached |
First stop paralytic, then sedative once patient shows motor activity or train of 4 on ulnar nerve stimulation |
Rewarming after cooling blankets ± ice: |
Remove cooling blankets (and ice if still in use) |
Rewarming after cooling vest use: |
Program device for controlled rewarming over 8–12 h. Dial in desired warming rate on machine, keep device in place and program for target temp of 37 °C (98.6 °F) for the next 48 h (72 h total). |
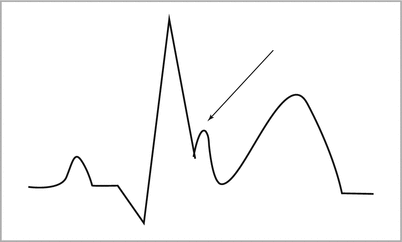
Figure 28-2
An Osborn wave on electrocardiogram
Neurophysiological Findings in Postanoxic Coma
Brain activity in comatose cardiac arrest patients is assessed using electroencephalography (EEG) recorded from electrodes placed on the scalp, and somatosensory evoked potentials (SSEP). Common EEG and SSEP findings are described in this section. Their quantitative prognostic significance is described in Tables 28-4 and 28-5 in the following section.
Table 28-4
False positive rates of univariate predictors of poor neurological outcomea
Predictor | Timing | FPR: No TH | FPR: TH |
|---|---|---|---|
Non-VF Cardiac arrest | 15 (6–30) % | ||
ROSC >25 min | 24 (13–40) % | ||
Low voltageb EEG – early on | Before TH | 47 (35–60) % < div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|