Chapter 52 Colon and Rectum
Embryology of the Colon and Rectum
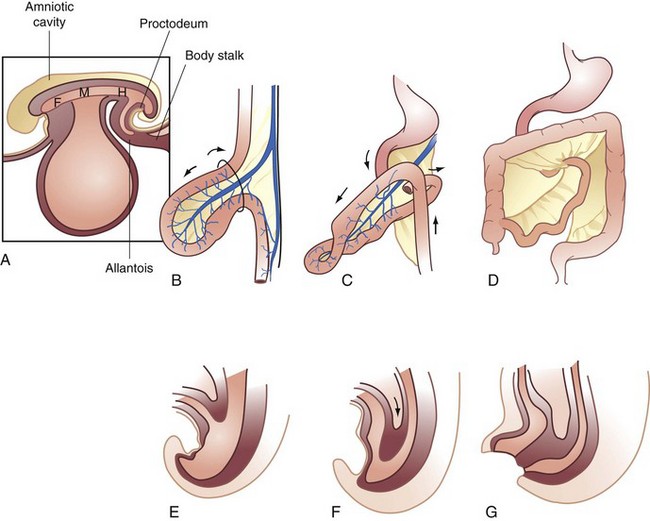
The endodermal roof of the yolk sac gives rise to the primitive gut tube. At the beginning of the third week of development, the gut tube is divided into three regions—the midgut, which opens ventrally, positioned between the foregut in the head fold and the hindgut in the tail fold. Development progresses through the stages of physiologic herniation, return to the abdomen, and fixation. The acquisition of length and formation of dedicated blood and lymphatic supplies takes place during this time (Fig. 52-1).
Anatomy of the Colon, Rectum, and Pelvic Floor
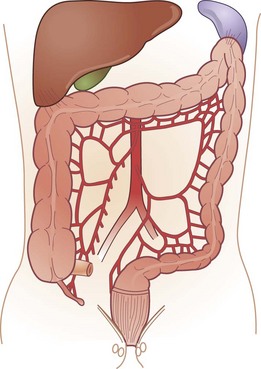
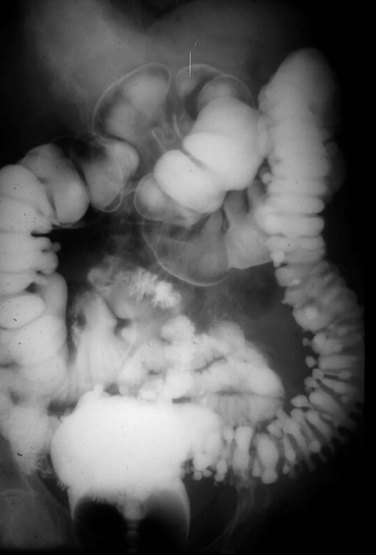
The colon and rectum constitute a tube of variable diameter approximately 150 cm in length. The terminal ileum empties into the cecum through a thickened, nipple-shaped invagination, the ileocecal valve. The cecum is a capacious saclike segment of the proximal colon, with an average diameter of 7.5 cm and length of 10 cm. Although it is distensible, acute dilation of the cecum to a diameter more than 12 cm, which can be measured by a plain abdominal radiograph, can result in ischemic necrosis and perforation of the bowel wall. Surgical intervention may be required when this degree of cecal distention is caused by obstruction or pseudo-obstruction (Fig. 52-2).
Pararectal Fascia
The endopelvic fascia is a thick layer of parietal peritoneum that lines the walls and floor of the pelvis. The portion that is closely applied to the periosteum of the anterior sacrum is the presacral fascia. The fascia propria of the rectum is a thin condensation of the endopelvic fascia that forms an envelope around the mesorectum and continues distally to help form the lateral rectal stalks. The lateral rectal stalks or ligaments are actually anterolateral structures containing the middle rectal artery. The stalks reside in close proximity to the mixed autonomic nerves, containing sympathic and parasympathetic nerves, and division of these structures close to the pelvic sidewall may result in injury to these nerves, resulting in impotence and bladder dysfunction (Fig. 52-3).
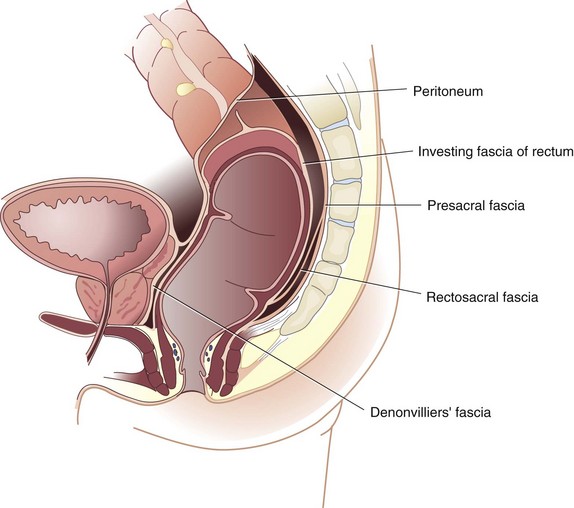
FIGURE 52-3 Endopelvic fascia.
(From Gordon PH, Nivatvongs S [eds]: Principles and practice of surgery for the colon, rectum and anus, ed 2, St Louis, 1999, Quality Medical Publishing, p 10.)
Pelvic Floor
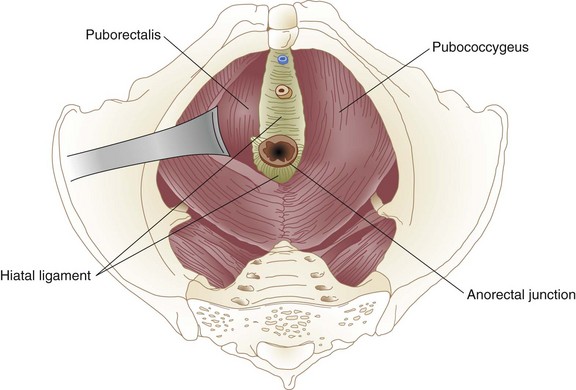
The muscles of the pelvic floor, like those of the anal sphincter mechanism, arise from the primitive cloaca. The pelvic floor, or diaphragm, consists of the pubococcygeus, iliococcygeus, and puborectalis, a group of muscles that together form the levator ani. The pelvic diaphragm resides between the sacrum, obturator fascia, ischial spines, and pubis. It forms a strong floor that supports the pelvic organs and, with the external anal sphincter, regulates defecation. The levator hiatus is an opening between the decussating fibers of the pubococcygeus that allows egress of the anal canal, urethra, and dorsal vein in men and the anal canal, urethra, and vagina in women. The puborectalis is a strong, U-shaped sling of striated muscle coursing around the rectum just above the level of the anal sphincters. Relaxation of the puborectalis straightens the anorectal angle and permits descent of feces; contraction produces the opposite effect. The puborectalis is in a state of continual contraction, a factor vital to the maintenance of continence. Puborectalis dysfunction is an important cause of defecation disorders. The pubococcygeus and iliococcygeus most likely participate in continence by applying lateral pressure to narrow the levator hiatus (Figs. 52-4 and 52-5).
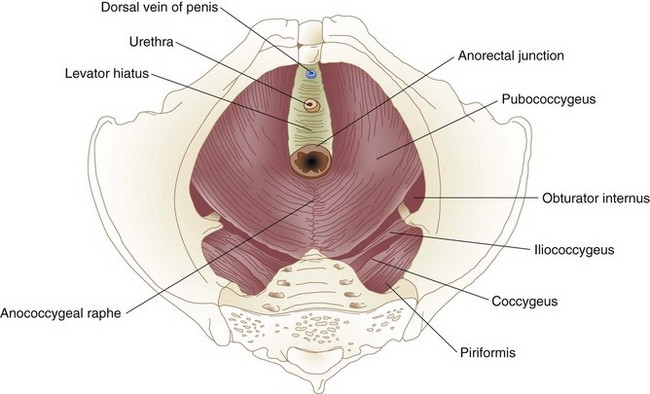
FIGURE 52-4 Levator muscles.
(From Gordon PH, Nivatvongs S [eds]: Principles and practice of surgery for the colon, rectum and anus, ed 2, St Louis, 1999, Quality Medical Publishing, p 18.)
Arterial Supply and Venous and Lymphatic Drainage
Knowledge of the embryologic development of the intestinal tract provides an excellent foundation for understanding the anatomic blood supply. The foregut is supplied by the celiac artery, the midgut by the SMA, and the hindgut by the IMA (Figs. 52-6 and 52-7). Anatomic redundancy confers survival advantages and, in the intestinal tract, this feature is provided by extensive communication between the major arteries and collateral blood supply (Fig. 52-8). The territory of the SMA ends at the distal portion of the transverse colon and that of the IMA begins in the region of the splenic flexure. A large collateral vessel, the marginal artery, connects these two circulations and forms a continuous arcade along the mesenteric border of the colon. Vasa recta from this artery branch off at short intervals and supply the bowel wall directly (Fig. 52-9). The SMA supplies the entire small bowel, giving off 12 to 20 jejunal and ileal branches to the left and up to three main colonic branches to the right. The ileocolic artery is the most constant of these branches; it supplies the terminal ileum, cecum, and appendix. The right colic artery is absent in 2% to 18% of specimens; when present, it may arise directly from the SMA or as a branch of the ileocolic or middle colic artery. It supplies the ascending colon and hepatic flexure and communicates with the middle colic artery through collateral marginal artery arcades. The middle colic artery is a proximal branch of the SMA. It generally divides into right and left branches, which supply the proximal and distal transverse colon, respectively. Anatomic variations of the middle colic artery include complete absence in 4% to 20% and the presence of an accessory middle colic artery in 10% of specimens. The left branch of the middle colic artery may supply territory also supplied by the left colic artery through the collateral channel of the marginal artery. This collateral circulation in the area of the splenic flexure is the most inconsistent of the entire colon and has been referred to as a watershed area, vulnerable to ischemia in the presence of hypotension. In some studies, up to 50% of specimens were found to lack clearly identified arteries in a small segment of colon at the confluence of the blood supplies of the midgut and hindgut. These individuals rely on adjacent vasa recta in this area for arterial supply to the bowel wall. In practice, surgeons avoid making anastomoses in the region of the splenic flexure, fearing that the blood supply will not be sufficient to permit healing of the anastomosis, a situation that could lead to anastomotic leak and sepsis.
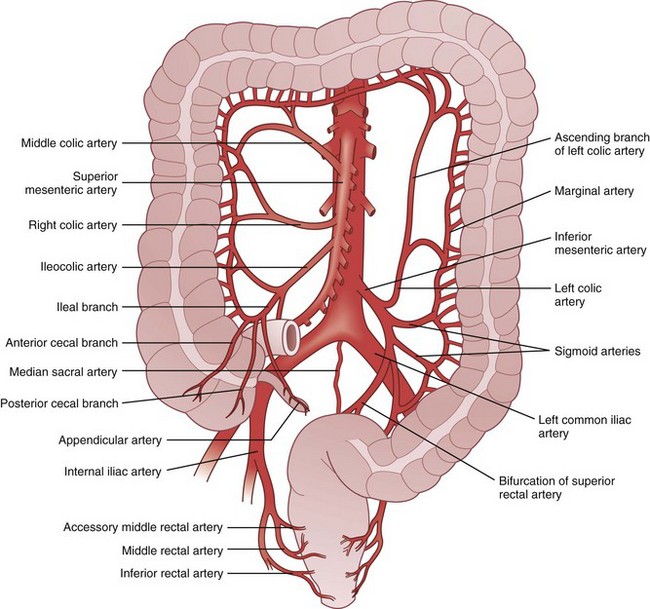
FIGURE 52-6 Arterial supply of the colon.
(From Gordon PH, Nivatvongs S [eds]: Principles and practice of surgery for the colon, rectum and anus, ed 2, St Louis, 1999, Quality Medical Publishing, p 23.)
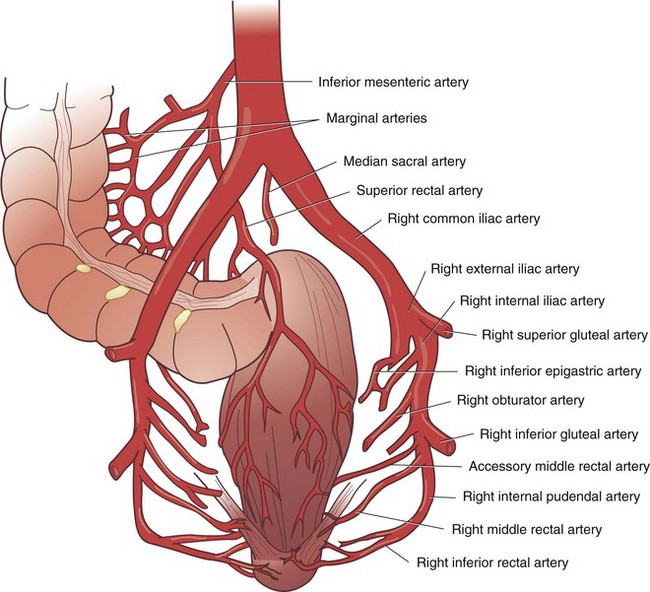
FIGURE 52-7 Arterial supply of the rectum.
(From Gordon PH, Nivatvongs S [eds]: Principles and practice of surgery for the colon, rectum and anus, ed 2, St Louis, 1999, Quality Medical Publishing, p 24.)
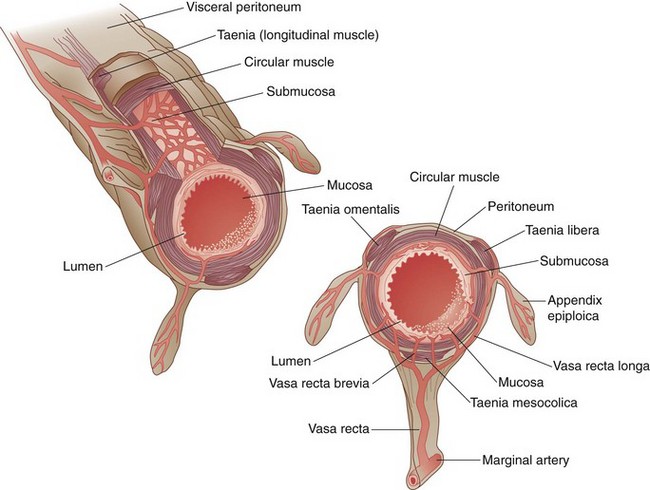
FIGURE 52-9 Cross-sectional anatomy of the colon, with vasa brevia and vasa recta.
(From Gordon PH, Nivatvongs S [eds]: Principles and practice of surgery for the colon, rectum and anus, ed 2, St Louis, 1999, Quality Medical Publishing, p 26.)
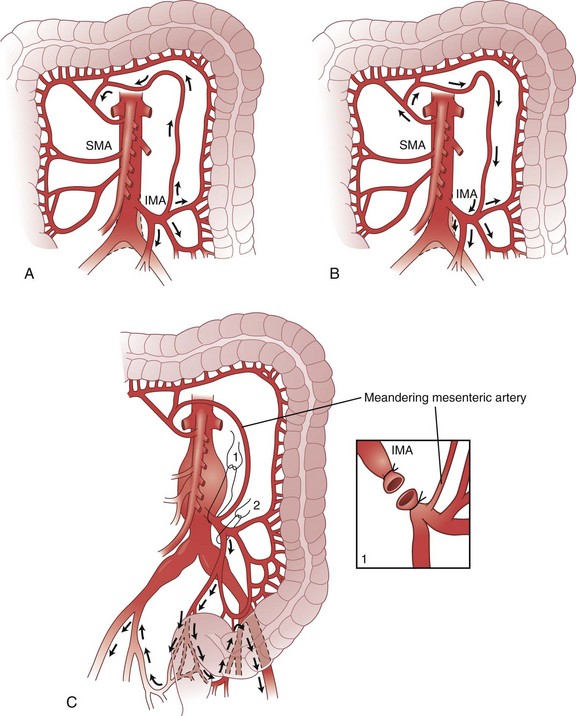
The arc of Riolan is a collateral artery, first described by Jean Riolan (1580-1657), that directly connects the proximal SMA with the proximal IMA and may serve as a vital conduit when one or the other of these arteries is occluded. It is also known as the meandering mesenteric artery and is highly variable in size. Flow can be forward (IMA stenosis) or retrograde (SMA stenosis), depending on the site of obstruction. Such obstruction results in increased size and tortuosity of this meandering artery, which may be detected by arteriography; the presence of a large arc of Riolan thus suggests occlusion of one of the major mesenteric arteries (Fig. 52-10).
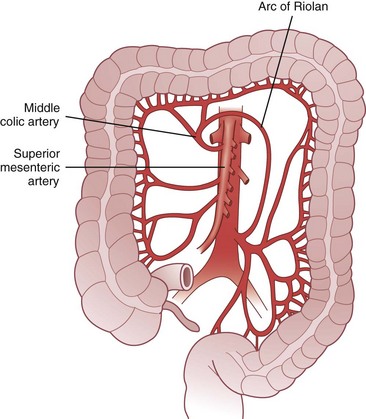
FIGURE 52-10 Arc of Riolan.
(From Gordon PH, Nivatvongs S [eds]: Principles and practice of surgery for the colon, rectum and anus, ed 2, St Louis, 1999, Quality Medical Publishing, p 27.)
The venous drainage of the colon and rectum mirrors the arterial blood supply. Venous drainage from the right and proximal transverse colon empties into the superior mesenteric vein, which coalesces with the splenic vein to become the portal vein. The distal transverse colon, descending colon, sigmoid, and most of the rectum drain into the inferior mesenteric vein, which empties into the splenic vein to the left of the aorta. The anal canal is drained by the middle and inferior rectal veins into the internal iliac vein and subsequently the inferior vena cava. The bidirectional venous drainage of the anal canal accounts for differences in patterns of metastasis from tumors arising in this region (Fig. 52-11).
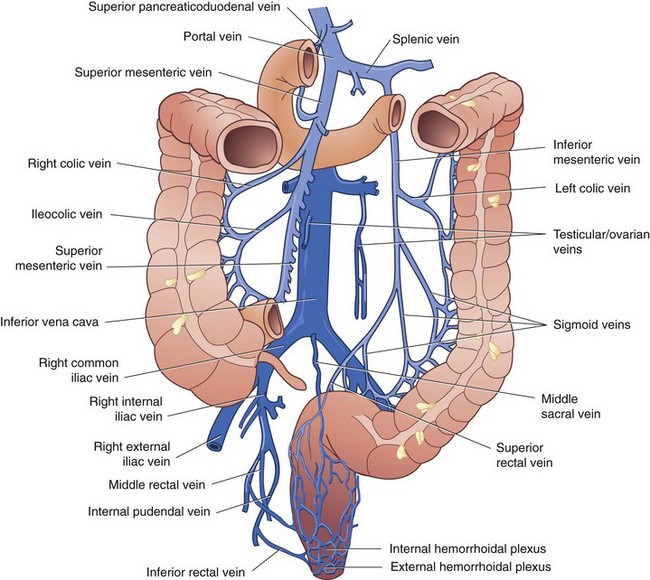
FIGURE 52-11 Venous drainage of the colon and rectum.
(From Gordon PH, Nivatvongs S [eds]: Principles and practice of surgery for the colon, rectum and anus, ed 2, St Louis, 1999, Quality Medical Publishing, p 30.)
Lymphatic drainage also follows the arterial anatomy. The wall of the large bowel is supplied with a rich network of lymphatic capillaries that drain to extramural channels paralleling the arterial supply. Lymphatics from the colon and proximal two thirds of the rectum ultimately drain into the para-aortic nodal chain, which empties into the cisterna chyli. Lymphatics draining the distal rectum and anal canal may drain to the para-aortic nodes or laterally, through the internal iliac system, to the superficial inguinal nodal basin. Although the dentate line roughly marks the level where lymphatic drainage diverges, classic studies by Block and Enquist using dye injection demonstrated that spread through lymphatic channels occurs to adjacent pelvic organs, such as the vagina and broad ligament, when injections are administered as high as 10 cm proximal to the dentate line (Figs. 52-12 and 52-13).
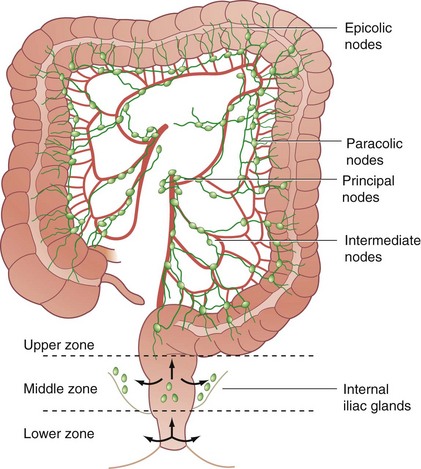
FIGURE 52-12 Lymphatic drainage of the colon.
(From Corman ML [ed]: Colon and rectal surgery, ed 4, Philadelphia,1998, Lippincott-Raven, p 21.)
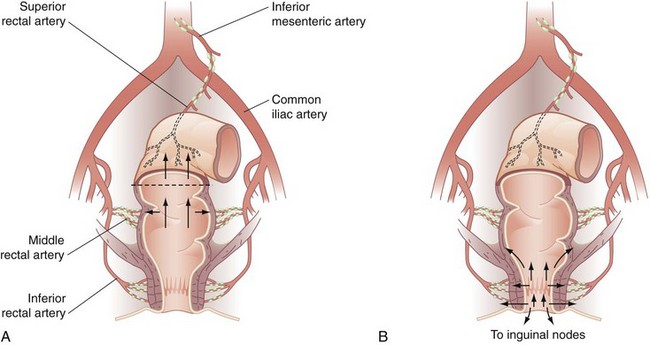
FIGURE 52-13 Lymphatic drainage of the rectum (A) and anal canal (B).
(From Gordon PH, Nivatvongs S [eds]: Principles and practice of surgery for the colon, rectum and anus, ed 2, St Louis, 1999, Quality Medical Publishing, p 32.)
Physiology of the Colon
Recycling of Nutrients
Colonic Flora
Nutrients are digested within the intestinal lumen with the aid of biliopancreatic and GI secretions. By the time the chyme reaches the terminal ileum, most of the nutrients have been absorbed, leaving a succus entericus composed of electrolyte-rich fluid, bile salts, and some proteins and starches that have resisted digestion. An enormous quantity of autochthonous flora, consisting of more than 400 bacterial species, resides in the large intestine. Large bowel contents may contain as many as 1011 to 1012 bacterial cells/g, contributing approximately 50% of fecal mass.1 Most these colonic species are anaerobes. These bacteria feed on proteins sloughed from the bowel wall and undigested complex carbohydrates.
Colonic microflora provide several important functions to the host, including barrier functions that help maintain epithelial integrity, nutritive functions that utilize plant polysaccharides, and developmental functions that stimulate epithelial cell differentiation and angiogenesis and, finally, immune functions via the gut. Gut associated lymphoid tissue contributes to both innate and adaptive immunity.1 Short-chain fatty acids (SCFAs) are produced by microbial breakdown and fermentation of dietary starches. These fatty acids are the principal source of nutrition for the colonocyte. Bacteroides species predominate throughout the colon, comprising two thirds of the total counts of the proximal colon and almost 70% of the bacteria in the rectum. Escherichia, Klebsiella, Proteus, Lactobacillus, and enterococci are the predominant species of facultative anaerobes.
Prebiotics and Probiotics
Probiotics can be defined as dietary supplements that contain live cultures of bacteria and yeast that are beneficial to colonic and host function. The two most widely used agents are Lactobacillus and Bifidobacterium. Recent studies have indicated that probiotics may have widespread health benefits, including stimulation of immune function, anti-inflammatory effects, and suppression of enteropathogenic colonization.2 In addition, they may increase the digestibility of dietary proteins, enhance absorption of amino acids, and play a protective or therapeutic role against Clostridium difficile–associated diarrhea.3 The ultimate role of probiotics has not yet been determined. There is conflicting data in regard to whether they work more effectively as primary therapy or as prophylaxis against recurrent C. difficile–associated diarrhea. Indications for their use are evolving, but may include necrotizing enterocolitis in neonates, patients with HIV-AIDS, and neutropenic patients undergoing chemotherapy. Further research is needed, but the evidence for probiotic usage in various settings is encouraging.
Short-Chain Fatty Acids
The primary end products of bacterial fermentation are SCFAs. Absorption of SCFAs in the large intestine is efficient; only 5% to 10% are lost in the feces. The three primary fatty acids produced are acetate, propionate, and butyrate in a ratio of 3 : 1: 1. SCFAs have key roles in colonic and also overall human metabolism. They are metabolized in three main sites: (1) colonocytes use butyrate as their primary energy source; (2) hepatocytes metabolize all three SCFAs to various degrees for use in gluconeogenesis; and (3) muscle cells oxidize acetate to generate energy. Metabolism of the SCFAs can provide up to 70% of colonocyte energy needs, reduce glucose oxidation, and spare other essential amino acids for metabolism.4 SCFAs also influence GI motility via the ileocolonic brake mechanism, which is defined as the inhibition of gastric emptying and nutrients reaching the ileocolonic junction.
Motility
Colonic motility patterns may be more simply divided into two primary patterns, segmental activity and propagated activity. Segmental activity consists of single contractions or rhythmic bursts of contractions. The purpose of these segmental contractions is to propel fecal matter distally via a directed pressure gradient toward the rectum in discrete distances and allow for mixing, which promotes optimal absorption. The second pattern is propagated activity, commonly classified on the basis of amplitude as low-amplitude or high-amplitude propagated contractions.5 High-amplitude propagated contractions have been historically referred to as mass movements, or migrating motor complexes whose role is shifting large quantities of contents through the colon. These have an important role in defecation, with mass movements propelling larger volumes of fecal matter to the distal colon and emptying of the descending colon into the sigmoid colon and rectum. Little is known about low-amplitude propagated contractions, but they are associated with distention of the viscous and passage of flatus.
Diverticular Disease
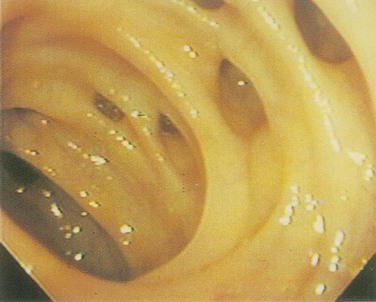
A diverticulum is an abnormal sac or pouch protruding from the wall of a hollow organ, which is, for the purposes of this discussion, the colon. A true diverticulum is composed of all layers of the intestinal wall, whereas a false diverticulum, or pseudodiverticulum, lacks a portion of the normal bowel wall. The diverticula that commonly occur in the human colon are protrusions of mucosa through the muscular layers of the intestine. Because these mucosal herniations are devoid of the normal muscular layers, they are pseudodiverticula (Fig. 52-14).
Pathogenesis
Diverticula are actually herniations of mucosa through the colon at sites of penetration of the muscular wall by arterioles. These sites are on the mesenteric side of the antimesenteric taeniae. In some cases, the arteriole penetrating the wall can be displaced over the dome of the diverticulum. This close relationship between the artery and diverticulum is responsible for the massive hemorrhage that can occasionally complicate diverticulosis (Fig. 52-15).
There is often a striking hypertrophy of the muscular layers of the colonic wall associated with diverticulosis. This thickening of the colonic wall, usually affecting the sigmoid colon, may precede the appearance of diverticula. Diverticula most commonly affect the sigmoid colon and are confined to the sigmoid in approximately 50% of patients with diverticulosis. The next most common area involved is the descending colon (≈40% of affected individuals), and the entire colon has diverticula in 5% to 10% of patients with diverticulosis. Even in patients with diverticula involving the entire colon, the muscular thickening characteristic of the disease is usually confined to the sigmoid (Fig. 52-16).
It has long been conjectured that factors contributing to diverticular disease, or at least diverticulitis, is the consumption of nuts, popcorn, and small seeds, such as are found in tomatoes, and patients with diverticular disease are often counseled to avoid these foods. However, a large prospective study of men without known diverticular disease has failed to detect an increase in the risk of diverticulosis or diverticular complications.11
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree