Fig. 43.1
Timeline of medical device development ©2013 Heart Valves: From Design to Clinical Implantation, Clinical trial requirements for cardiac valves, Iaizzo JC, Lovas ATF. With kind permission of Springer Science+Business Media, New York
With the diversity of cardiac devices, there are various types of clinical trials that can be defined, from trials where a novel valve technology is being used for the first time (first in human studies) to post-market trials in which a cardiac therapy has obtained regulatory approval but is studied further to examine long-term effects, pursue additional indications, and/or to obtain more specific information about the overall therapy. In other words, clinical evidence is vital not only to demonstrate the safety and efficacy of a device/therapy in humans but also to further examine how well the device works compared to standard of care, other devices, and/or concomitant treatments. In the specific case of a newly developed heart valve, studies will often be designed to compare the new valve against the native valve, other heart valve devices, and/or the current standard of care treatments. Using the example of planning a clinical trial for a new heart valve, this chapter provides a general summary of the present state of clinical trials, including an overview of (1) the current stance of regulatory bodies that oversee trials, (2) specific features of a trial design, and (3) the many considerations involved in the proper implementation of heart valve clinical trials.
Regarding the design of a clinical trial, the following groups/individuals may be identified, each with their specific role(s) (Fig. 43.2):
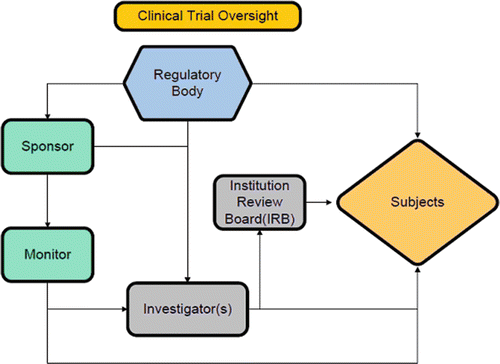

Fig. 43.2
Clinical trial oversight ©2013 Heart Valves: From Design to Clinical Implantation, Clinical trial requirements for cardiac valves, Iaizzo JC, Lovas ATF. With kind permission of Springer Science+Business Media, New York
Sponsor(s): The developer of the technology seeking approval for market release.
Investigator(s): Non-biased individuals that will implant/deploy the novel technology and will also be responsible for individual patient follow-ups. In some cases, investigators can also develop their own field clinical trial(s).
Monitor(s): Individuals responsible to ensure that the trial is performed in an ethical and proper fashion. They usually work for the sponsor and make frequent visits to participating institutions to review data and regulatory documents. Regulatory bodies also have their own process for auditing sponsors and investigators through their Bioresearch Monitoring group(s).
Institutional Review Board (IRB)/Ethics Committee (EC): The overseeing body at a given institution that is ultimately responsible for ensuring that the clinical protocol is appropriate and that the institutional investigators perform the study in a proper and ethical manner. These boards may have different names according to the institutional structure.
Subjects: Individual patients who were deemed appropriate to be enrolled (meeting all inclusion criteria and none of the exclusion criteria) into the planned clinical trial and who provided informed consent to participate.
43.2 Regulatory Bodies
Regulations and the regulatory bodies that govern both cardiac devices and clinical trials play important roles in how new technologies reach the market. A solid partnership between a sponsor and a regulatory body, aided by clear communication, can affect whether the technology can reach the market in an expeditious manner. Regulatory bodies are important, as they ensure consistency in clinical trials and that they are run properly in order to provide the supportive scientific evidence required. Specifically, there are numerous regulatory bodies that provide oversight for cardiac device clinical trials throughout the world. A brief overview of the regulatory bodies from three different countries follows, yet our discussion focuses mainly on the Food and Drug Administration (FDA) in the United States.
43.2.1 Food and Drug Administration (United States)
The FDA is responsible for regulating medical devices and therefore oversees the associated clinical trials exclusively within the United States. The FDA’s mission statement consists of two primary parts: (1) promoting public health by promptly and efficiently reviewing clinical research and taking appropriate action on marketing of regulated products in a timely manner and (2) protecting public health by ensuring a reasonable assurance of safety and effectiveness of devices intended for human uses [1]. The Center for Devices and Radiological Health (CDRH) is the branch that oversees medical devices. Cardiac devices that incorporate other therapies (i.e., pharmacological agents) will need to confirm if they will work through CDRH and/or the Center for Drug Evaluation and Research (CDER).
In the United States, there are three regulatory classes of devices based on the considered levels of risk involved. All class I–III devices are subject to general controls, meaning the FDA reviews factors such as labeling, registrations, etc. Class I devices have the lowest amount of risk and regulatory controls (devices such as elastic bandages and surgical gloves). Class II devices must meet specific performance standards in addition to all class I requirements (devices such as surgical drapes). Most stringently, class III devices require premarket approval (PMA) to ensure their safety and efficacy. As such, class III devices are considered as the riskiest category of devices and include devices such as implantable pacemakers and heart valves.
It should be noted that the FDA regulations for medical device products are detailed in Title 21 of the Code of Federal Regulations (CFR). The most applicable parts of CFR 21 that apply to cardiac devices and clinical trials include Part 812 (Investigational Device Exemption, or IDE) and Part 814 (PMA). Most new and novel cardiac devices are required to undergo IDE clinical trials before receiving FDA approval. As the regulatory landscape is typically in constant flux, it is crucial to reference and follow current guidance and regulations set forth by the respective governing regulatory body.
In the example of heart valves, there is specific guidance in documents like the FDA’s Heart Valves—IDE and PMA Applications Draft Guidance; these documents state that “a replacement heart valve is a device intended to perform the function of any of the heart’s natural valves” [2]. A replacement heart valve is defined as a pre-amendment-type device, that is, a device marketed prior to passage of the Medical Device Amendments to the Federal Food, Drug, and Cosmetic Act (the Act).
Furthermore, this FDA draft explains that clinical trials are necessary to evaluate most new replacement heart valve designs, and it also recommends that clinical investigations are executed by following the methods described in ISO 5840:2005 or an equivalent document. Specifically, the document ISO 5840:2005 is a guide for cardiovascular implants and valve prostheses provided by the International Organization for Standardization (ISO) [3]. When developing a clinical database and trial strategy, the appropriate FDA guidance should be referenced.
43.2.2 Other Regulatory Bodies
In Europe there are various notified bodies that provide oversight of clinical trials. The most prevalent regulatory oversight applies to the 27 countries in the European Economic Area; these are countries required to obtain a CE mark (Conformité Européenne or European Conformity). Importantly, the criteria to receive a CE mark in Europe are notably different than those for securing FDA approval. As mentioned previously, to receive approval for a new technology in the United States, the manufacturer must demonstrate the device to be reasonably safe and effective. To receive approval to release a device to market in the European Union, the manufacturer must demonstrate that the medical device is safe and that it performs in a manner consistent with the manufacturer’s intended use [4]. Interestingly, given these differences in geographic regulatory approval, most manufacturers typically seek approval in Europe or other countries before the United States. Moving into other countries poses different obstacles which may influence the intended quality and importance of every clinical trial completed for the new device.
43.2.3 Good Clinical Practice Oversight
Similar to the importance of following Good Manufacturing Practice (GMP) and Good Laboratory Practice (GLP) when prototyping cardiac devices, it is important to follow guidelines for how to appropriately conduct clinical studies that could affect the safety and well-being of human participants. Good Clinical Practice (GCP) was developed by a collaborative group of regulatory authorities worldwide, including the European Union, Japan, and the United States by the International Conference on Harmonisation. Effective in 1997, GCP provides international assurance that data and results of clinical investigations are credible and accurate and that the rights, safety, and confidentiality of participants in clinical research studies are respected and protected. More specifically, GCP consists of 13 principles which are detailed in Table 43.1.
Ethics |
1. Ethical conduct of clinical trials |
2. Benefits justify risks |
3. Rights, safety, and well-being of subject prevail |
Protocol and science |
4. Nonclinical and clinical information supports the trial |
5. Compliance with a scientifically sound, detailed protocol |
Responsibilities |
6. Institutional Review Board/Independent Ethics Committee approval prior to initiation |
7. Medical care and decisions by qualified physicians |
8. Each individual qualified (education, training, experience) to perform his/her tasks |
Informed consent |
9. Freely given from every subject prior to participation |
Data quality and integrity |
10. Accurate reporting, interpretation, and verification |
11. Protects confidentiality of records |
Investigational products |
12. Conform to Good Manufacturing Practice and used per protocol |
Quality control/quality assurance |
13. Systems with procedures to ensure quality of every aspect of the trial |
43.3 The Generalized Clinical Trial Cycle/Process
Addressing all aspects of a clinical trial in depth is an enormous undertaking and beyond the scope of this chapter; thus, the following sections will highlight some of the foundational methods and processes of a typical heart valve clinical trial which can generally be translated to the complexities of other cardiac devices. As you can see in Table 43.2, there are many tasks that need to be addressed with the development and execution of a clinical trial. It is important to note that some of these tasks may occur simultaneously.
Table 43.2
Standardized clinical research process
1. Prepare a clinical plan |
2. Recruit investigators |
3. Prepare protocol |
4. Prepare case report forms |
5. Prepare informed consent form |
6. Perform investigator site visit |
7. One-on-one investigator reviews, including clinical plan, protocol, case report forms, and informed consent form |
8. Obtain an investigator agreement |
9. Obtain IRB approvals for each participating institution |
10. File an IDE |
11. Obtain IDE approval |
12. Perform periodic investigator meetings |
13. Conduct the clinical study, i.e., a multicenter study |
14. Monitor the multicenter study |
15. Conclude study |
16. Compile data from each institution |
17. Analyze overall collected data |
18. Write final clinical report |
43.3.1 Features of a Trial Design for a Newly Developed Cardiac Device
It is pertinent to research and understand all current published information and relevant heart valve trial data prior to planning and executing a clinical trial. There is much to be gained from studying the details of previous trial designs, as well as the subsequent outcomes associated with those trials. In regards to gaining FDA approval for any cardiac device, the importance of clinical evidence cannot be stressed enough. In the beginning stages of planning a clinical trial design, associated publications and previous research may help shape important components for the new trial, such as: patient inclusion/exclusion criteria, statistical designs employed in such trials, and/or the general patient populations to be studied.
A well-controlled clinical investigation includes a clear objective and defined methods of analysis. More specifically, the objectives should address the proposed medical claims for the investigational device and these objectives should be refined to explicitly address the safety and efficacy of the heart valve in a defined population. Next, it is important to structure a trial so there can be a valid comparison to controls. For example, in current transcatheter valve therapy trials, the new therapy (transcatheter valves) is directly compared to a standard open-heart valve surgery. A control group gives the results a meaningful comparison to an existing therapy or treatment, which is important to the scientific community and may be crucial for future marketing. Often, an appropriate control group can be identified by performing a careful and thorough literature search or seeking out the key opinion leaders in the related field. Furthermore, performing early research on the specific disease or conditions that the heart valve will treat is equally important, in order to understand the natural progression of the disease or condition and the current benefits or limitations of other treatments. It should be noted this step is often completed in earlier phases of device prototyping, but it is recommended that designers review the research once again just prior to planning the clinical trial. Finally, literature searches on similar treatments/heart valves can also assist in identifying the appropriate disease populations and justifying the inclusion and exclusion criteria for the trial.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


