Fig. 19.1
Management protocol for patients with asymptomatic carotid bruit or nonhemispheric symptoms. MRA magnetic resonance angiography, CEA carotid endarterectomy, st. stenosis
If stenosis of ≥60–99% was detected and the patient is a good risk, a magnetic resonance angiogram/CTA can be done to complement the findings of the ultrasound, and if confirmed, a CEA is recommended for patients at high risk for stroke. If the magnetic resonance angiogram/CTA was not conclusive or contradicted the ultrasound findings, then angiography may be considered in centers with a minimal stroke risk rate from angiography. However, in patients with a good quality carotid DUS, an endarterectomy may be considered based on ultrasound findings only. Several authorities would not recommend CEA in asymptomatic patients unless carotid stenosis exceeds 70–80%. In patients who had a good quality ultrasound showing total occlusion, no further follow-up is needed. However, if the quality of the ultrasound was limited, a magnetic resonance image/CTA is recommended to confirm occlusion.
Another indication for studying asymptomatic patients is to screen patients with advanced coronary artery disease or peripheral vascular diseases. Due to the diffuse nature of atherosclerosis, many of these patients have occult carotid bifurcation lesions with a resulting increased risk of stroke. This type of screening is carried out most often in patients who are being considered for cardiac or major peripheral arterial operations in order to detect carotid stenoses that may substantially increase the risk of intraoperative and postoperative stroke. Carotid screening is covered in depth in Chap. 12.
Patients with Atypical or Nonhemispheric Symptoms
Patients with atypical or nonhemispheric symptoms often do not have a clear indication for angiography. Some of these patients’ symptoms include dizziness, blackouts, bilateral visual disturbances, or bilateral motor or sensory deficits. Since a variety of nonvascular causes, such as orthostatic hypotension, cardiac arrhythmias, and medications, may be responsible for these symptoms, noninvasive carotid testing is important in identifying these patients with hemodynamically significant carotid stenosis. Our management protocol for this group of patients is outlined in Fig. 19.1.
Patients with Focal Neurologic Deficits (Transient Ischemic Attacks or Strokes)
A major proportion of transient ischemic attacks (TIAs) or permanent focal neurologic deficits in hemispheric distribution or with amaurosis fugax is caused by embolization from ulcerations and atheromatous plaques. Therefore, the purpose of carotid screening in patients with hemispheric neurologic symptoms is to identify lesions that could be the source of cerebral emboli or could reduce cerebral hemispheric blood flow. In the North American Symptomatic Carotid Endarterectomy Trial (NASCET) study [21], carotid endarterectomy was highly beneficial for patients with recent hemispheric TIAs or mild strokes and >70–99% and 50–69% stenoses of the ipsilateral internal carotid artery. Based on these results, patients with symptoms of severe stenoses of the carotid artery should be treated by CEA unless their medical condition makes the risk of surgery prohibitive. Our management protocol for this group of patients is outlined in Fig. 19.2. As noted in Fig. 19.2, the initial step is to obtain a color DUS, and if the study is diagnostic and shows <50% stenosis, the patient is treated medically (e.g., antiplatelet therapy, statins, and risk modifications and repeat color DUS in 12 months). If the stenosis is ≥50%, the ultrasound is of good quality, and the patient has classical focal hemispheric symptoms, a CEA can be done based on the carotid DUS findings alone; or MRA/CTA can be done to complement the ultrasound findings, and if the diagnosis is confirmed, surgery may be considered without angiography. Angiography is reserved for patients with a marginal quality DUS or magnetic resonance angiogram or in patients with contradictory magnetic resonance angiogram and DUS results. If the DUS shows total occlusion and the ultrasound was of good quality, no further workup is usually necessary. For patients with a DUS that is not diagnostic, magnetic resonance angiogram/CTA is done, and if it is diagnostic and the severity of stenosis is established, surgery can be done accordingly. If the MRA/CTA is not diagnostic, angiography is recommended.
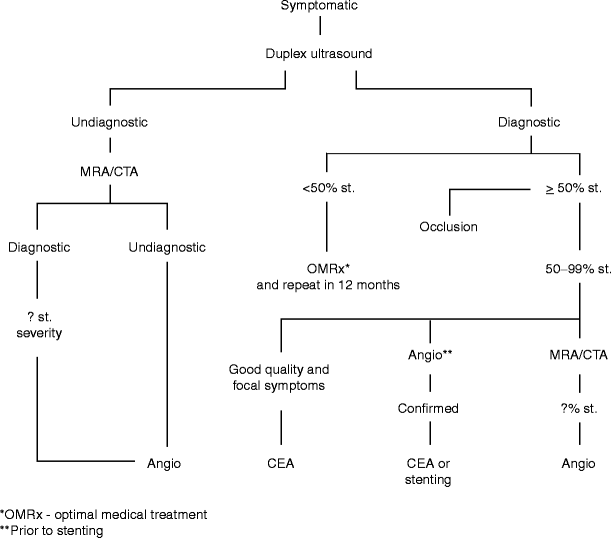

Fig. 19.2
Management protocol for patients with suspected hemispheric symptoms (cerebrovascular disease). For <50% stenosis, repeat ultrasound in 12 months. MRA magnetic resonance angiography, CEA carotid endarterectomy, OMRx optimal medical treatment, st. stenosis
Specific Duplex Criteria for Specific Clinical Situations
In choosing our criteria for peak systolic velocity and end diastolic velocity, we chose the values that gave the highest overall accuracy. However, which criteria to use should depend on the “outcome” desired by the clinician. Although some surgeons have advocated CEA based on duplex criteria alone [5, 22, 23], the decision to proceed with an arteriogram is based on the duplex findings in the majority of patients. The mortality and morbidity of arteriography vary from institution to institution, but can be significant [6, 24]. We propose that vascular laboratories at institutions with significant mortality and morbidity in relation to carotid arteriography use duplex criteria with 95% or greater PPV and the best overall accuracy in order to minimize the number of patients undergoing unnecessary arteriography (Table 19.1). These criteria can also be utilized when CEA is performed without preoperative arteriography. In those institutions where arteriography does not significantly add to the mortality and morbidity of the overall treatment of carotid disease, we suggest using the criteria described in Table 19.2. These criteria have the highest negative predictive value to ensure that only a minimum number of patients with equal to or greater than 60% or 70% stenoses are missed.
Table 19.1
Selected optimal criteria with best PPV (≥95%) and overall accuracy in detecting ≥60–99% and 70–99% ICA stenosis
PPV (%) | Overall accuracy (%) | Sensitivity (%) | Specificity (%) | NPV (%) | |
|---|---|---|---|---|---|
Best PPV for ≥ 60% ICA stenosis | |||||
ICA PSV ≥ 220 cm/s | 96 | 82 | 64 | 98 | 76 |
ICA EDV ≥ 80 cm/s | 96 | 87 | 79 | 97 | 84 |
ICA/CCA PSV ratio ≥ 4.25 | 96 | 71 | 41 | 99 | 65 |
ICA PSV and EDV 150 and 65* | 96 | 90 | 82 | 97 | 86 |
Best PPV for ≥ 70% ICA stenosis | |||||
ICA PSV ≥ 300 cm/s | 97 | 80 | 48 | 99 | 76 |
ICA EDV ≥ 110 cm/s* | 100 | 91 | 75 | 100 | 87 |
ICA/CCA PSV ≥ none | – | – | – | – | – |
ICA PSV and EDV 150, 110* | 100 | 91 | 75 | 100 | 87 |
Table 19.2
Selected optimal criteria with best NPV (≥95%) and overall accuracy in detecting ≥60–99% and 70–99% ICA stenosis
NPV (%) | Overall accuracy (%) | Sensitivity (%) | Specificity (%) | PPV (%) | |
|---|---|---|---|---|---|
Best NPV for ≥ 60% ICA stenosis | |||||
ICA PSV ≥ 135 cm/sa | 99 | 80 | 99 | 64 | 71 |
ICA EDV – none | – | – | – | – | – |
ICA/CCA PSV ratio ≥ 1.62 | 95 | 71 | 97 | 47 | 62 |
ICA PSV and EDV – none | – | – | – | – | – |
Best NPV for ≥ 70% ICA stenosis | |||||
ICA PSV >150 cm/sa | 99 | 80 | 99 | 69 | 65 |
ICA EDV ≥ 60 cm/s | 96 | 83 | 94 | 77 | 71 |
ICA/CCA PSV ≥ none | – | – | – | – | – |
ICA PSV and EDV – none | – | – | – | – | – |
A duplex classification was proposed by us which would consist of lesions <30% stenosis, ≥30–49% stenosis, ≥50–59% stenosis, ≥60–69% stenosis, and ≥70% stenosis. This new duplex classification would fit into the existing trials [NASCET, ACAS, and Veteran’s Administration Cooperative Study (VA)], and may be of benefit as new conclusions are released [25]. By reporting results using these criteria, the clinician will be better able to make decisions regarding the need for CEA or arteriogram based on the risks and benefits for individual patients. With the added risks of arteriography, decisions to operate would be better based on duplex findings alone. Having PPVs of 90–97% and accuracies of 87–93% can eliminate many unnecessary arteriograms. For those vascular laboratories who may be using the consensus duplex criteria, specific velocities can be used accordingly, as indicated in Chap. 7.
It is important to note that the data obtained by individual vascular laboratories will vary as a result of differences in equipment, abilities, and consistencies of vascular technicians and reader interpretations [25]. Therefore, each laboratory must adapt a method that employs the equipment they use and validate their method when using proposed new duplex criteria.
Intraoperative Duplex Ultrasound Assessment of Carotid Endarterectomy
Intraoperative use of the B-mode ultrasound imaging system for completion evaluation of the CEA has been advocated by Sigel et al. [26] The development of smaller scanning heads and probes together with techniques of sterilization has made this application feasible. The ultrasound examination can be performed quickly and, unlike angiography, requires no delay for film processing. Nor is it necessary to inject contrast material. Angiography is also associated with the risks of subintimal injections, thromboembolic complications, and allergic reactions.
Despite careful operative techniques, certain vascular defects can be missed, for example, intimal flaps, luminal thrombus/platelet aggregation, and stricture, that occur in the course of carotid repair (Fig. 19.3). These defects can escape visual inspection and palpation of the repair. If these defects are left undetected, they can result in stroke secondary to thrombus formation, platelet aggregation, or arterial thrombosis, or they may result in postoperative recurrent carotid stenoses. Blaisdell et al. reported the fallibility of clinical assessment by routine completion angiography, which revealed unsuspected defects in 25% of cases [27]. A number of investigators have subsequently confirmed the observations of Blaisdell et al. by using angiography, alone or in combination with various ultrasound techniques, such as continuous-wave Doppler examination, pulse Doppler spectral analysis, or duplex ultrasonography. Intraoperative monitoring has consistently documented severe defects in the internal carotid artery (ICA) or the common carotid artery (CCA) that warranted immediate correction in approximately 2–10% of all repairs [28–34]. Although the percentage of patients with residual repair defects in whom a postoperative stroke would develop if the defects were left untreated is not known, prudent surgical practice dictates that detection and revision of these defects should be done at the primary operation, since the sequelae of an ICA thrombosis are frequently catastrophic.

Fig. 19.3
The application of Doppler probe to detect defects of a repaired internal carotid artery (intimal flap)
Baker et al. [30] reported that recurrent stenoses (>75%) developed in 17% of patients with abnormal unrepaired CEAs by intraoperative imaging compared with 4.3% in normal CEAs (p < 0.001). This suggests that abnormalities detected by intraoperative DUS, if not corrected, may contribute to recurrent carotid stenosis after CEA. Kinney et al. [31] showed the importance of intraoperative scanning in a prospective study of 461 CEAs. They correlated the results of intraoperative assessment by clinical inspection, ultrasound, or arteriography with an end point of stroke. The CEA site was assessed by ultrasound and arteriography in 268 cases, by ultrasound and Doppler spectral analysis alone in 142 cases, and with clinical inspection in only 51 cases. Based on intraoperative assessment, 26 endarterectomies (6%) were revised at the time of the surgery. Perioperative morbidity was similar in cases with normal, mildly abnormal, or no ultrasound. There were 12 temporary (3%) and six permanent (1%) neurologic deficits and six deaths (four strokes and two cardiac events). Based on life-table analysis, the incidence of >50% ICA stenosis or occlusions was increased (p < 0.007) in patients with residual flow abnormality or no study. However, patients with normal intraoperative studies had a significantly lower rate of late ipsilateral stroke compared with the other patients (p = 0.04). The incidence of stroke was increased (p = 0.00016) in patients with ICA restenosis or occlusion (3 of 35) compared with patients without recurrent stenosis (3 of 426) during a mean follow-up of 30 months. It was concluded that a normal intraoperative duplex scan may prevent recurrent stenosis as well as stroke after CEA in the long term.
Most authorities rely on imaging or Doppler flow detection technique to exclude technical defects. The diagnostic signal analysis is highly sensitive and specific (>90%), particularly if pulse Doppler analysis is performed. This technique is simple, widely available, and relatively inexpensive. Although abnormalities of the Doppler flow signal are readily apparent by audible interpretation, quantitative spectral analysis is preferable. With flow- and pressure-reducing lesions, a spectral broadening is present throughout the pulse cycle, and a peak systolic velocity (PSV) exceeding 150 cm/s is noted. Visual inspection of velocity spectra and calculation of PSV can be obtained by a high-frequency pulse Doppler probe or duplex scanning, which permits classification of flow patterns into three categories: normal flow, mild to moderate flow disturbance, and severe flow disturbance [28]. When a significant residual flow abnormality is identified, angiography is usually recommended to delineate the abnormality before reexploration of the repair.
Recently, intraoperative duplex ultrasonography has been advocated because of its ability to provide both anatomic and hemodynamic information [32–35]. Improvements in linear ray scan head design and electronic signal processing, including color-coded velocity display, have made duplex scanning feasible in the operating room and an ideal modality for intraoperative assessment of CEA. Duplex scanning has an advantage over Doppler flow analysis alone, in that the structure of the anatomic defects associated with severe flow disturbance can usually be determined. B-mode imaging is sensitive in detecting small intimal defects in flaps; however, most authorities have not repaired these minor lesions, and the outcome of the procedure has not been adversely influenced [34].
A comparison of intraoperative and early postoperative duplex findings after CEA indicated that a majority of these abnormalities identified by duplex scanning within 3–6 months of CEA represent residual rather than recurrent stenoses [33].
Recently, Ascher et al. [36] reported on the value of intraoperative carotid artery duplex scanning in a modern series of 650 consecutive primary endarterectomy procedures (April 2000–April 2003). Major technical defects at intraoperative duplex scanning (>30% luminal ICA stenosis, free-floating clot, dissection, arterial disruption with pseudoaneurysm) were repaired. CCA residual disease was reported as wall thickness and percent stenosis (16–67%; mean 32% ± 8%) in all cases. Postoperative 30-day TIA, stroke, and death rates were analyzed. There were no clinically detectable postoperative thromboembolic events in this series. All 15 major defects (2.3%) identified with duplex scanning were successfully revised. These included seven intimal flaps, four free-floating clots, two ICA stenoses, one ICA pseudoaneurysm, and one retrograde CCA dissection. Diameter reduction ranged from 40% to 90% (mean, 67 ± 16%), and peak systolic velocity ranged from 69 to 497 cm/s (mean, 250 ± 121 cm/s). Thirty-one patients (5%) with the highest residual wall thickness (>3 mm) in the CCA and 19 (3%) with the highest CCA residual diameter reduction (>50%) did not have postoperative stroke or TIA. Overall postoperative stroke and mortality rates were 0.3% and 0.5%, respectively; combined stroke and mortality rate was 0.8%. One stroke was caused by hyperperfusion, and the other occurred as an extension of a previous cerebral infarct. It was concluded that intraoperative duplex scanning had a major role in these improved results because it enabled detection of clinically unsuspected significant lesions. Residual disease in the CCA does not seem to be a harbinger of stroke or TIA.
Color duplex scanning with a 7.5- to 10-MHz linear ray transducer has been used for intraoperative studies.
These studies are conducted with the transducer covered by a sterile disposable plastic sleeve that contains acoustic gel (Fig. 19.4). The probe is generally positioned in the cervical incision directly over the exposed carotid repair. A sterile solution is instilled into the incision for acoustic coupling. As the surgeon scans the arterial repair, the technologist adjusts the instrument to optimize the Doppler angle, sample volume, color-coded image, and recorded velocity spectra. Vessel walls are imaged at 90°, but blood flow patterns should be evaluated at Doppler angles of <60°.


Fig. 19.4
Transducer covered by sterile disposal plastic sleeve that contains acoustic gel
For CEAs with primary closure, the entire CEA segment should be examined with duplex ultrasound. The point in the CCA at which the lesion is transected should be examined. Normally, this should leave a distinct shelf, which can be appreciated on B-mode imaging. This can be easily visualized in both transverse and longitudinal views. The velocity data proximal to, in, and distal to the endarterectomy site should also be done in longitudinal view, and sampling of the PSVs in the endarterectomy site should be obtained. Similarly, scanning of the proximal ICA in the bulb and beyond it should be done, and attention should be called to the point of the transaction of the plaque or the end of the plaque distally. The external carotid artery should also be examined for the first few centimeters, looking for residual plaques or areas of thrombus. In patients who have CEAs closed with a patch, either polytetrafluoroethylene (PTFE) or Dacron, it is impossible to scan through the patch itself because of the air within the wall of the patch. However, it is possible to scan along the side of the artery, either posterior or anterior to the patch, which may yield the necessary information (Figs. 19.5 and 19.6).
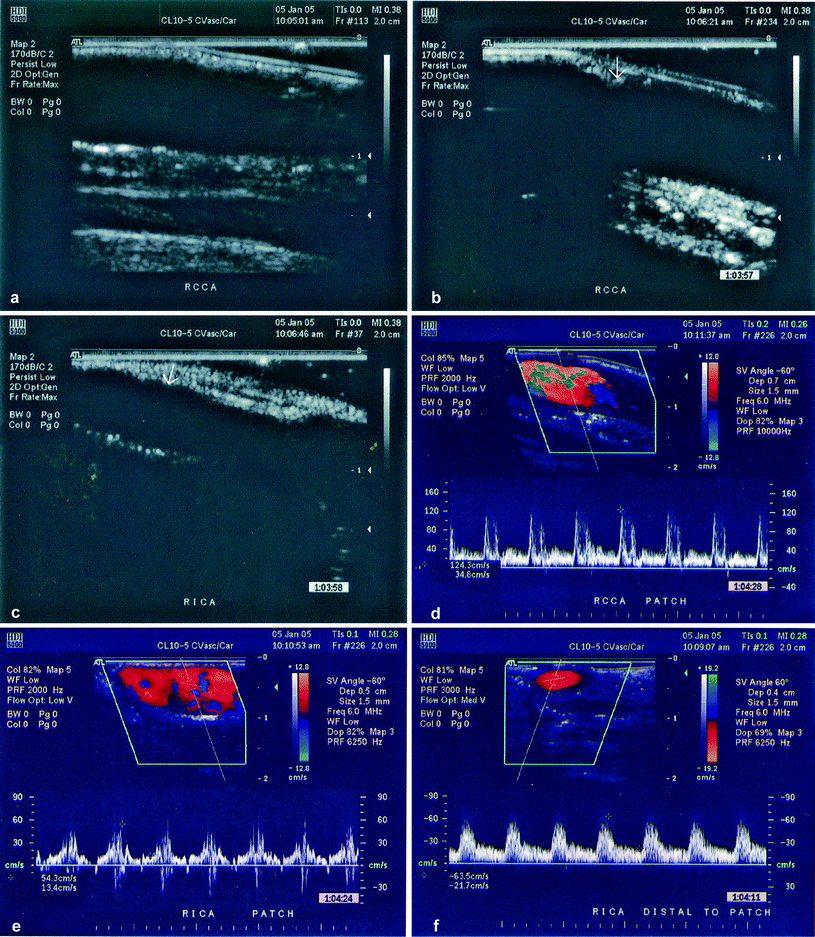

Fig. 19.5
Probe scanning position for carotid endarterectomy closed with a patch. It is impossible to scan through PTFE patch, but operator can scan along the side of artery, either posterior or anterior to the patch

Fig. 19.6
Intraoperative duplex ultrasound of carotid endarterectomy: (a) common carotid artery in gray scale. Note the shelf of the proximal end of carotid endarterectomy, (b) common carotid artery bifurcation in gray scale, (c) internal carotid artery in gray scale, (d) common carotid artery with color flow, (e) internal carotid artery with patch with color flow, (f) distal internal carotid artery with color flow
The limitations of this technique are largely related to lack of experience, correct measurement of duplex derived flow velocities, recognition of abnormal flow patterns, and transducer size. Intraoperative duplex imaging has the following advantages over angiography: comparable or higher accuracy, safety, ease of repeated use after reexploration, and low cost. Color duplex scanning is also sensitive to variations in anatomy and minor vascular defects that may alter blood flow streamlines. Certain flow patterns produced by carotid patch angioplasty should be noted and should not be regarded as abnormal. Some authorities have reported vascular defects in as many as one-third of their repairs, but only one-third of these appear to justify reexploration. Further details can be seen in Chap. 16.
Intraoperative Monitoring of Carotid Endarterectomy with Transcranial Doppler Sonography
Transcranial Doppler (TCD) sonography has the advantage of allowing monitoring of both hemodynamic and embolic events, primarily in the middle cerebral artery distribution during CEA. One of the common uses of TCD is intraoperative monitoring to determine whether shunting is necessary and whether the shunt is malfunctioning [37]. TCD can be a useful indicator that early carotid clamping is necessary if multiple emboli were detected [37]. TCD can also be helpful in patients where shunts are not being used since a TCD signal will give an idea of the flow through the middle cerebral artery. The middle cerebral artery cannot be insonated in 5–15% of patients, most commonly because of the lack of a window for Doppler signal penetration of the skull. Severe cerebral ischemia is considered present in the first minute after carotid occlusion if the middle cerebral artery velocity decreases to 15% of the baseline or lower and mild ischemia if it drops to 15–40% of the baseline. An adequate perfusion is present if the velocity is >40% of the baseline [38]. Following insertion of the shunt or upon declamping, a brisk recovery in middle cerebral artery velocity should be seen, usually >80%. Absolute mean velocities of 15 cm/s or even 30 cm/s have been alternatively suggested. A middle cerebral artery velocity of 30 cm/s has correlated roughly with a carotid artery stump blood pressure of 50 mmHg. Some authorities reported that TCD detects critically low flow that results in neurologic deficits, even in the absence of electroencephalographic changes. The converse is also true: a pronounced drop in mean velocity has been observed in conjunction with a normal EEG and no resultant cerebral infarction, the cortex surviving from the other cerebral and leptomeningeal vessels.
In a recent study, Ackerstaff et al. [39] concluded that in CEA, TCD-detected microemboli during dissection and wound closure, ≥90% middle cerebral artery velocity decrease at cross clamping, and ≥100% pulsatility index increase at clamp release are associated with operative stroke. In combination with the presence of preoperative cerebral symptoms and ≥70% ipsilateral ICA stenosis, these four TCD monitoring variables can reasonably discriminate between patients with and without operative stroke. This supports the use of TCD as a potential intraoperative monitoring modality to alter the surgical technique by enhancing a decrease of the risk of stroke during or immediately after the operation.
TCD can also be used in the postoperative period to detect early thrombosis of the carotid artery, continued embolization, or the hyperperfusion syndrome. Another useful indication for TCD monitoring is in the early postoperative period since more than one-half of patients develop emboli in the first 3 h after carotid endarterectomy [40], and a majority of these will stop without further treatment; however, if the TCD indicates an increasing number of these emboli, treatment may be necessary (e.g., dextran infusion). TCD can also be useful in postoperative monitoring by measuring the middle cerebral artery velocities. If these velocities decrease, it may indicate compromising of the carotid endarterectomy site, meanwhile increasing velocities may be indicative of hyperperfusion syndrome. Presently, there is no level 1 evidence that TCD is essential in the routine practice of carotid artery surgery.
It has been reported that markedly increased mean velocity (150% of the baseline) may herald an intracranial hemorrhage. The use of TCD monitoring during CEA has led some surgeons to modify their operative techniques based on hearing a distressing frequency of emboli while operating with the continuously audible TCD. Further details on TCD are discussed in Chap. 10.
Patients Who Develop a Neurologic Deficit After Leaving the Operating Room
If patients wake up well after CEA and then develop a neurologic deficit, emergent reexploration is indicated. If the deficit proves to be a TIA as symptoms resolve prior to the return to the operating room, heparin anticoagulation followed by duplex scan is preferred. A thrombosed ICA may be treated operatively or medically (anticoagulation), particularly in patients with dense deficits. A patent carotid without apparent pathology is immediately followed by brain CT/CTA to identify intracranial hemorrhage or other pathology and assess the intracranial vasculature. If negative, oral anticoagulation is started. Thromboembolism of inaccessible intracranial vasculature has been treated with selective catheterization and lytic therapy, although this is still considered investigational. Blood clots found at the endarterectomy site are treated by emergency reexploration.
Post-carotid Endarterectomy Surveillance
Restenosis is a known entity that occurs after CEA and may vary between 12% and 36%, but the frequency of restenosis varies depending on the diagnostic method used and the frequency of follow-up examinations [41–52]. Several studies have reported on the value of postoperative carotid duplex surveillance, but no consensus has been reached [41–51]. The advantages that have been cited are detection of significant restenosis prior to the onset of neurologic events, which aids in the prevention of potential strokes, and follow-up on the contralateral carotid artery to document the development of surgically correctable stenosis. Opponents of routine postoperative carotid duplex surveillance claim that restenosis is benign in nature; therefore, a large number of strokes may not be prevented by surveillance [44, 45, 47, 48, 51].
Despite the high rate of restenosis, symptoms attributed to restenoses are rare; therefore, several authorities have suggested that routine surveillance of patients after CEA is not efficacious [43, 45, 48].
Several factors were associated with restenosis: continued smoking, small internal carotid artery diameter, operative defect detected at intraoperative assessment, and primary closure after CEA. Moore et al. [53] prospectively determined the incidence of restenosis using Doppler ultrasound follow-up to 5 years in ACAS patients who underwent CEA. The aggregate incidence of residual and recurrent carotid stenosis for all time intervals was 13%. Early restenosis (<2 years) in this group of patients was found in 8% and late restenosis in 2%. Of the 136 patients who were felt to have restenosis, only 8 (5.9%) underwent reoperation, only one of whom was for symptoms. There was also no correlation between late stroke and recurrent stenosis. Similarly, Cao et al. [54] randomized 1,353 who underwent CEA using the eversion technique (678) or standard CEA (primary closure in 419 and patch closure in 256). The life-table estimate of the cumulative risk of restenosis at 4 years was 4% in the eversion CEA group and 9% in the standard CEA group. Almost all of these patients (98%) were asymptomatic.
Mattos et al. [45] described their experience with postoperative carotid duplex surveillance and found an equal stroke-free survival at 5 years between patients with and without >50% restenosis. In addition, only one of 380 patients suffered a stroke in their study, suggesting a benign clinical significance of recurrent carotid artery stenosis. Mackey et al. claim a low rate of clinically significant restenosis [44]. Their retrospective series of 258 patients (348 arteries) show a potential 4% incidence of late strokes, but this included all patients who underwent repeat CEA for asymptomatic restenosis. They also noted that the majority of restenoses (53%) remained asymptomatic and did not progress to occlusion throughout follow-up. Of 10 documented late occlusions, eight did not result in stroke. Eight patients with operable restenosis had TIAs and underwent reoperation. They found that even patients with 75–99% restenosis most often remained asymptomatic (37%) or had TIAs (32%). Only two (11%) of 19 patients with 75–99% restenosis had an unheralded stroke. They felt that postoperative carotid duplex surveillance was not justified due to the low incidence of symptomatic restenosis.
In spite of these findings, investigators have been reluctant to advise that postoperative carotid duplex surveillance be abandoned because the cost-effectiveness of this surveillance has not been formally investigated. Others have reported that high-grade stenosis (>75%), whether caused by myointimal hyperplasia of the CEA site or progressive atherosclerosis of the contralateral carotid artery, is associated with an increased risk of late stroke [31, 51].
Ouriel et al. reported an 11% incidence of restenotic lesions greater than 80%. Although the incidence of symptoms with restenotic lesions was low (12%), the onset of symptoms at the time of occlusion was significant [46]. Forty-two percent of patients became symptomatic at the time of occlusion, with 33% resulting in a stroke. This led to the observation that critical restenoses are precursors to stroke, even if asymptomatic, and, therefore, the detection of >80% restenosis allows future stroke prevention if operative intervention is undertaken [46]. Mattos et al. also described the outcome for >80% restenosis. In their group, one of three patients with >80% restenosis suffered a stroke, one had a TIA, and one remained asymptomatic. This suggests a more serious course once restenosis reaches >80% [45].
So far, a consensus has not yet been reached in the surgical literature regarding the usefulness, cost-effectiveness, or timing of postoperative carotid duplex surveillance.
Timing of Postoperative Carotid Duplex Surveillance
Several authors have recommended an initial surveillance duplex on the operative carotid system within the first 6 months [42, 45–47, 51] to detect residual stenosis from the operative procedure or early restenosis [46]. For example, Roth et al. [51] recently recommended an initial DUS to ensure a technically successful CEA, with subsequent postoperative carotid duplex surveillance at 1–2 years, as long as restenosis and contralateral stenoses remain <50%. More frequent follow-up (every 6 months) is warranted if >50% stenosis is noted, or with the onset of symptomatic disease [51].
Several studies have reported that the majority of restenoses occurs during the first 1–2 years after CEA. Mattos et al. [42] noted that 70% of restenoses were detected within 1 year after the CEA, and 96% developed within 15 months. Thomas et al. [41] reported that 70% of restenoses in their study occurred within 1 year of the CEA. Similar observations were noted by us previously [49].
Ricco et al. [55] reported on the need for follow-up duplex scan 1 year after CEA was performed with prosthetic patching and intraoperative completion arteriography. A total of 605 CEA procedures with prosthetic patch closure and intraoperative completion arteriography were performed in 540 patients. All patients underwent duplex scan at 4 days and then yearly after the procedure. Intraoperative completion arteriography showed abnormalities in 114 cases, including 17 involving the ICA and 73 involving the external carotid artery. Successful revision was achieved in all cases and confirmed by repeat arteriography. Postoperative duplex scans at 4 days detected three abnormalities involving the ICA (0.5%), including asymptomatic occlusion in one case and residual stenosis >50% in two cases. Ninety-eight percent of patients were stenosis-free at 1 year. Actuarial stroke-free survival was 98.3% at 3 years. Diameter reduction of the contralateral carotid artery progressed over 70% within 1 year after CEA in 22.9% of patients with contralateral carotid stenosis over 50% at the time of the initial intervention. The findings of this study indicate that duplex scan follow-up 1 year after CEA with intraoperative completion arteriography is unnecessary unless postoperative duplex scan demonstrates residual stenosis of the ICA. However, duplex scan at 1 year is beneficial for patients presenting with contralateral carotid artery disease with diameter reduction >50% at the time of CEA.
Lovelace et al. [56] conducted a study on optimizing duplex follow-up in patients with an asymptomatic ICA stenosis of <60%. All patients who underwent initial carotid duplex examination for any indication since January 1, 1995, with at least one patent, asymptomatic, previously nonoperated ICA with <60% stenosis; with 6 months or greater follow-up; and with one or more repeat duplex examinations were entered into the study. On the basis of the initial duplex examination, ICAs were classified into two groups: those with a PSV <175 cm/s and those with a PSV of 175 cm/s or more. Follow-up duplex examinations were performed at varying intervals to detect progression from <60% to 60–99%. A total of 407 patients (640 asymptomatic ICAs with <60% stenosis) underwent serial duplex scans (mean follow-up, 22 months). Three ICAs (0.5%) became symptomatic and progressed to 60–99% ICA stenosis at a mean of 21 months, whereas four other ICAs occluded without stroke during follow-up. Progression to 60–99% stenosis without symptoms was detected in 46 ICAs (7%) (mean, 18 months). Of the 633 patent asymptomatic arteries, 548 ICAs (87%) had initial PSVs <175 cm/s and 85 ICAs (13%) had initial PSVs of 175 cm/s or more. Asymptomatic progression to 60–99% ICA stenosis occurred in 22 (26%) of 85 ICAs with initial PSVs of 175 cm/s or more, whereas 24 (4%) of 548 ICAs with initial PSVs <175 cm/s progressed (p < 0.0001). The Kaplan–Meier method showed freedom from progression at 6 months, 12 months, and 24 months was 95%, 83%, and 70%, respectively, for ICAs with initial PSVs of 175 cm/s or more versus 100%, 99%, and 95%, respectively, for ICAs with initial PSVs <175 cm/s (p < 0.0001).
They concluded that patients with <60% ICA stenosis and PSVs of 175 cm/s or more on initial duplex examination are significantly more likely to progress asymptomatically to 60–99% ICA stenosis, and progression is sufficiently frequent to warrant follow-up duplex studies at 6-month intervals. Patients with <60% ICA stenosis and initial PSVs <175 cm/s may have follow-up duplex examinations safely deferred for 2 years.
Cost-Effectiveness of Postoperative Carotid Duplex Surveillance
There have been reports that postoperative carotid duplex surveillance is not cost-effective since there is such a low incidence of symptomatic restenosis. Patel et al. evaluated the cost-effectiveness of postoperative carotid duplex surveillance [50]. They concluded that postoperative carotid duplex surveillance after CEA has an unfavorable cost-effectiveness ratio. In the process of their analysis, they identified a subset of patients in which postoperative carotid duplex surveillance may be cost-effective. These included patients in whom the rate of progression to >80% stenosis exceeded 6% per year. In their analysis, they felt that some groups of patients could potentially have a rate of disease progression that approaches or exceeds the level at which postoperative carotid duplex surveillance becomes cost-effective. Some of these include patients with multiple risk factors, for example, smoking, hypertension, hyperlipidemia, diabetes mellitus, coronary artery disease, female gender, and young age. In addition, they concluded that with postoperative carotid duplex surveillance, the rate of carotid artery occlusion could be reduced by 15% per year. Our evaluation of the cost of postoperative carotid duplex surveillance agrees with these conclusions. Three hundred and ninety-nine CEAs were randomized into 135 with primary closure, 134 with PTFE patch closures, and 130 with vein patch closures and followed for a mean of 47 months. Postoperative carotid duplex surveillance was done at 1, 6, and 12 months and every year thereafter (a mean of 4.0 studies/artery). A Kaplan–Meier analysis was used to estimate the rate of ≥80% restenosis over time and the time frame of progression from <50% to 50–79% and ≥80% stenosis.
Greater than or equal to 80% restenosis developed in 24 (21%) with primary closure and 9 (4%) with patching. A Kaplan–Meier estimate of freedom from 50% to 79% restenosis at 1, 2, 3, 4, and 5 years was 92%, 83%, 72%, 72%, and 63% for primary closure and 99%, 98%, 97%, 97%, and 95% for patching. A Kaplan–Meier estimate of freedom from ≥80% restenosis at 1, 2, 3, 4, and 5 years was 92%, 83%, 80%, 76%, and 68% for primary closure and 100%, 99%, 98%, 98%, and 91% for patching (p < 0.01).
Out of 56 arteries with 20–50% restenosis, 2/28 patch closures and 10/28 primary closures progressed to 50–<80% restenosis (p = 0.02) and 0/28 patch closures and 6/28 primary closures progressed to ≥80% (p = 0.03). In primary closures, the median time to progression from <50% to 50–79%, <50% to ≥80%, and 50–79% to ≥80% was 42, 46, and 7 months, respectively. Of the 24 arteries with ≥80% restenosis in primary closures, 10 were symptomatic. Thus, assuming that symptomatic restenosis would have undergone duplex examinations anyway, there were 14 asymptomatic arteries (12%) that could have been detected only by postoperative carotid duplex surveillance (estimated cost of $139,200) and would have been candidates for redo CEA. Of the nine arteries with patch closures (three PTFE and six vein patch closures) with ≥80% restenosis, six asymptomatic arteries (four vein patch closure and two PTFE, 3%) could have been detected by postoperative carotid duplex surveillance. In patients with a normal duplex at the first 6 months, only 4/222 (2%) patched arteries (two asymptomatic) developed ≥80% restenosis versus 5/13 (38%) in patients with abnormal duplex examinations (p < 0.001).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


