Chapter 60
Chronic Venous Insufficiency
Deep Vein Valve Reconstruction
Michael C. Dalsing
Chronic venous insufficiency (CVI) can be asymptomatic or advance to a state of chronic, unrelenting venous ulceration. Skin changes such as hyperpigmentation consistent with CVI of the lower extremity have been reported in 6 to 7 million U.S. citizens, with progression to venous ulceration occurring in up to 2%.1–3 It is estimated that the cost of venous ulcer care alone is more than $1 billion annually.4 The excellent early success of ulcer healing with compression is marred by the risk of recurrence in up to 40% of compliant patients but approaching 100% in noncompliant patients.5,6 Treating all superficial and perforator vein insufficiency can reduce the recurrence risk, but it still is noted in up to 33% of patients with primary deep CVI and 70% of those with the postthrombotic syndrome. These facts demonstrate the importance of deep venous disease in the pathophysiologic process of CVI.7 Deep venous obstructive disease in combination with venous insufficiency has been noted in up to 55% of patients with CVI, especially in those with the most severe symptoms.8 Correcting iliac obstruction while ignoring the deep venous insufficiency can result in a long-term ulcer-free rate of nearly 60%.9 What patients remain are a select but finite cohort with chronic deep venous insufficiency who will benefit from valve reconstruction.
Pathogenesis
Etiology
Rarely patients are observed with congenital absence of lower extremity venous valves, venous aplasia or dysplasia.10 More commonly patients with primary venous insufficiency have either floppy, redundant, elongated valve cusps or an enlarged venous diameter, both of which prevent normal valve cusp apposition.11–14 In these cases, there is no apparent inciting event that caused the anatomic changes observed. As a consequence of these pathologic changes, spontaneously with standing or with additional stresses, reflux occurs in the affected valves. Except in the case of aplasia or dysplasia, in-situ tightening (repair) of the structurally intact valve can render it competent and functional.
Secondary venous insufficiency is often the result of acute deep venous thrombosis (DVT) and in 40% to 70% of all affected patients may be the cause of chronic deep venous valvular incompetence.11,15–17 The resulting inflammation and scarring with or without recanalization can cause foreshortening and fibrosis of the valve leaflets, small perforations, or valve adhesion and luminal narrowing.11 The valves are generally so damaged that in-situ repair is not feasible. The options remaining to prevent complete system reflux are transplantation of a competent valve from a distant location, transposition of the incompetent venous system to a position distal to a local competent valve, or the use of less traditional valve substitutes.
This standard differentiation between “primary” and “secondary” (postthrombotic) venous disease has been challenged by clinical imaging studies and by direct observation of the veins and valves at surgery. The simultaneous occurrence of both pathologic processes within the same patient is a clinical observation made decades ago.18 During attempted surgical repair of venous valve reflux, 6 of 11 excised veins demonstrated typical postthrombotic pathologic changes, but nonthrombotic phlebosclerosis was noted in the remaining 5.11 Phlebosclerosis was defined as vein wall thickening or fibrosis at the valve station or thickening of the valve cusps or intima (or both). One theory suggested to explain the thickened yet preserved normal valve architecture is that primary reflux with the associated sustained high venous wall pressure might cause the pathologic picture. DVT could also be the cause with rapid resolution of venous thrombi, an event documented by investigators studying acute DVT,19 allowing the involved valves to escape the severe damage usually associated with thrombosis. Alternatively, the valve itself may not be directly involved in the thrombotic process. Venous reflux has been documented proximal to an isolated distal thrombosis.19–21 In this case, the fibrotic process in the vein wall may involve the valve station only during thrombus resolution; the resultant thickened, noncompliant vein wall could cause the cusps to become floppy by virtue of a decrease in wall diameter.11 The valve remains architecturally intact, thin, and compliant and can be made competent by standard reefing of the cusps.
Hemodynamics
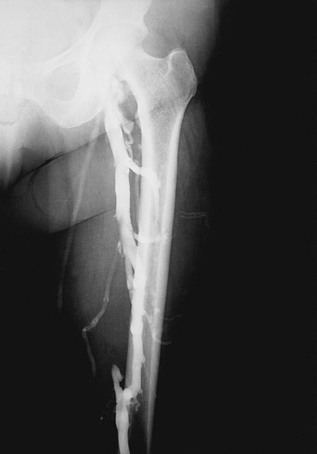
To realize the goal of controlling lower limb venous hypertension, correction of deep axial reflux proximal to the calf and ankle area is required (see Chapters 11 and 12). If one disregards this axiom, most commonly by ignoring profunda femoris vein reflux, isolated femoral vein valve reconstruction will be clinically unsuccessful because the offending hemodynamic effects will not be altered.22 Alternatively, when the profunda system is competent, repair of femoral vein incompetence does improve lower limb venous hemodynamics by preventing total axial reflux.22,23 In some cases of femoral vein thrombosis with occlusion, there is tremendous dilatation of the profunda femoris vein such that repair of valvular reflux in this vein will adequately reverse overall pathologic venous hemodynamics (Fig. 60-1).24 An alternative method of correcting significant axial reflux when both femoral systems are incompetent is to position a competent valve in the popliteal vein.25–27 At least one investigator has performed multiple tibial vein valve repairs to accomplish the same goal.28
Diagnostic Evaluation
History and Physical Examination
Patients with CVI may present initially with dependent edema, leg fatigue, itching, telangiectases, a feeling of overall fullness, or any combination thereof. Progression in severity may be noted by the presence of varicose veins, increasing pain, intractable edema, and skin changes (such as eczema, cellulitis, hyperpigmentation, lipodermatosclerosis, and stasis ulcers). A careful history should take note of previous episodes of DVT, known hypercoagulable conditions, and any limitations in physical and occupational activity resulting from venous insufficiency. In addition, the physical examination can eliminate other causes of the presenting signs and symptoms, such as ischemic ulcers, diabetic ulcers, dermatologic disorders, and even skin or soft tissue cancer. Each patient should ultimately be stratified according to the CEAP system to clearly define the venous disease present, which will allow the formation of a focused and appropriate management strategy.29 Candidates for deep venous valvular reconstruction typically have class 5/6 disease or disabling symptoms, such as severe edema (C4). An in-depth extension of the clinical classification (Venous Clinical Severity Score) provides a quantification of disease severity and is a useful evaluation tool to determine a patient’s response to treatment.30 Quality of life surveys provide an estimation of the impact of venous disease on the patient’s life and the effect that therapy has on the patient’s overall well-being.31 Application of these surveys before and after surgery will demonstrate those patients most improved by the intervention.
Noninvasive Evaluation
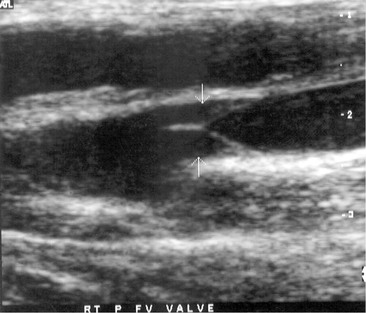
The noninvasive evaluation of patients with chronic deep venous insufficiency begins with a complete venous duplex study (see Chapter 18). It is essential to clarify the extent and anatomic location of all venous disease (specific veins can be imaged to determine the precise location of reflux), and it will provide some indication of the etiology (congenital, primary, or secondary) and aid in determining the pathophysiologic mechanism (reflux, obstruction, or both) of the disease present. The imaging is often so clear that venous valve cusps can be seen moving in the venous stream (Fig. 60-2). Insufficiency within any segment of the deep venous system is defined as a prolonged reflux time through the valve after a provocative test. A reflux time of longer than 0.5 second is considered abnormal in the tibial and deep femoral veins but is somewhat longer, more than 1 second, for the femoral and popliteal veins.32 Venous obstruction is seen as thickened, scarred, and constricted veins or valves with poor flow and diminished augmentation after distal or proximal compression. Respiratory variation can be lost as a consequence of local disease or proximal obstruction or stenosis. Similar imaging and spectral analysis will determine obstruction or, more commonly, insufficiency of the superficial and perforator veins. This is all the diagnostic evaluation needed if only the superficial and perforator veins are found to be pathologic.
I use air plethysmography (see Chapter 17) to provide an overall quantification of the impact of deep venous insufficiency before deep venous valvular reconstruction and to provide some insight into the effectiveness of treatment. Currently, this diagnostic modality is used for specific indications or as a research tool only.33
Invasive Evaluation
For patients being considered for deep venous valvular repair, venography is required for precise operative planning. Ascending venography (see Chapter 20) defines deep venous system anatomy, helps eliminate obvious obstruction as an etiologic factor, and provides a means of selecting the most disease-free portion of the incompetent and postthrombotic venous system when valve transplantation is an option.11,34 Ambulatory venous pressure has been shown to correlate with the presence of venous ulceration and is one method available to estimate the degree of venous hypertension.35 A venous reflux time of less than 20 seconds confirms that valvular reflux is present in the limb being evaluated. Intravenous hemodynamic study, although useful, can be provided by less invasive means, so it is not always a component of an ascending venographic evaluation. Similarly, the practice of directly measuring lower limb intravenous pressure before and after thigh cuff occlusion has not proved to be a reliable determinant of significant proximal venous occlusion.11,34,36 The importance of significant iliac vein occlusive disease is determined best by the use of intravenous ultrasonography.36 Descending venography (see Chapter 20) is used to determine valve leaflet integrity, anatomic location, and extent or degree of reflux (Fig. 60-3).37 Assessment of the competence of the profunda femoris venous system in addition to the femoral system is imperative because it will affect the choice and location of venous valve repair and the potential for valve transposition.27,38,39 Descending venography does not have absolute accuracy in determining the presence or absence of a normal valve.11 Raju and colleagues reported that descending venography had a false-positive rate of 11% (a valve considered present was absent at surgery) and false-negative rate of 25% (a valve thought absent was present on direct inspection) when direct inspection at operation was the “gold standard.”11 However, when it is combined with a well-performed venous duplex examination, it is the best method available to determine preoperatively whether in-situ reconstruction will be possible.
Treatment Selection
Natural History and Patient Risk Assessment
The natural history of patients being considered for deep venous valvular repair has generally been extensive because all less invasive interventions to solve the patient’s problems have been attempted and have failed. With the traditional approach of treating all superficial and perforator disease first regardless of the status of the deep system, about 33% of patients with primary deep venous incompetence and 70% of those with postthrombotic syndrome will experience ulcer recurrence, thus emphasizing that deep venous disease must eventually be treated in select patients.7 Separate investigators have added external valvuloplasty of the femoral vein to high ligation and saphenous vein stripping to improve the clinical durability in advanced disease, with gratifying results.40,41 Iliac vein occlusive disease has been treated with the knowledge that symptoms may resolve in about 60% of patients.9 For the remaining 40%, deep venous reflux will require intervention to alleviate symptoms.
Patient risk assessment must follow the tenets of any open operative intervention (see Chapter 31). Hemodynamic instability from the operation is minimal, but because the operative intervention is time-consuming and technically challenging, general anesthesia is typically required. An acceptable cardiac and pulmonary status is required. Systemic infection should be treated before intervention. Systemic disease that might have an impact on healing, such as collagen vascular disease, diabetes, chronic renal insufficiency, or lower extremity arterial occlusive disease, will influence the surgeon’s decision to perform a venous intervention.42 These conditions are relative contraindications to aggressive venous intervention unless they are corrected or medically optimized before venous valve repair.
Treatment Options
The treatment options available to correct deep venous valvular reflux are determined by the availability of complete and structurally intact venous valves. Selection of the operative technique when the valve cusps are architecturally preserved is somewhat determined by the degree of cusp prolapse, the surgeon’s preference, and the reported long-term success. In the case of valve prolapse secondary to vein wall dilatation only, vasospasm alone may reestablish a competent valve, and in such cases simple external banding of the vein will provide a workable solution to prevent reflux.43 When wall dilatation with more pronounced cusp prolapse is present, external valvuloplasty will provide a way to decrease wall diameter, and the use of certain techniques will also enable reefing of the valve cusps into a functional position. With pronounced valve leaflet prolapse, internal valvuloplasty allows direct and extensive cusp shortening and reduction of wall diameter. When there is no identifiable valve structure within an incompetent system, two potential options are available. If a parallel axial system has a competent valve or one that can be made so, a transposition procedure is an option. When this is not feasible, valve transplantation of a competent valve from a distant location remains an option. Valve substitutes are being investigated, with some autogenous options showing promise. The quest for a percutaneous option has provided few answers to date. In some instances, intervention in two or more separate axial venous systems (e.g., the femoral and profunda femoris veins) or into the popliteal location will be required to prevent axial reflux into the calf and ankle soft tissues. Multiple valve repairs in the same incompetent axial system (e.g., two or more in the femoral vein) are being investigated in the hope of decreasing recurrent reflux and thus symptoms28,44–46; however, many surgeons have reported success when only one valve per incompetent system is repaired.
Surgical Treatment
Relevant Anatomy
The common femoral, profunda femoris, and femoral veins are covered by skin, soft tissue, and fascia in the groin and by the sartorius muscle more distally. More caudally, the femoral vein occupies the adductor [Hunter’s] canal, which places it deep to the sartorius and flanked by the vastus medialis and adductor longus fascia muscles. As the femoral vein exits the adductor canal, it is called the popliteal vein and lies between the heads of the gastrocnemius muscle distally but is covered only by skin, soft tissue, and fascia directly behind the knee. The anterior tibial veins lie in the anterior calf compartment deep to the anterior tibialis muscle. The posterior tibial and peroneal veins lie in the deep posterior compartment and are covered by the soleus and gastrocnemius muscles, which form the superficial posterior compartment.47
Valves are universally present in the paired tibial and peroneal veins, with 3 to 12 valves rather evenly distributed in each vein.48 The majority of popliteal veins (>90%) have one to three valves with a slight trend to be located more caudal in the leg.48 The femoral veins have one to five valves.48,49 The most constant valve occurring in about 90% of patients is found in the femoral vein 1 to 2 cm distal to joining the profunda femoris vein.48–50 In 88% of patients, the profunda femoris vein has one to four valves.48 The common femoral vein may (30%-50% of patients) have one or two valves within a few centimeters of the inguinal ligament.48–50
Operative Planning
In the event that an intact valve has been confirmed by preoperative duplex and venographic studies, most experienced surgeons choose the proximal femoral vein for valvuloplasty or to repair both the femoral and profunda femoris valves if both systems are incompetent. This decision is based on the ease of exposure, familiarity with the approach, and size of the veins available for repair. When both proximal femoral axial systems are incompetent, some have chosen repair of the popliteal vein valve, which is considered the “gateway” to the lower leg venous system. However, mid or distal femoral vein valves can be repaired if they are surgically accessible and amenable to valvuloplasty by virtue of retained architecture. As one proceeds down the venous system, vein diameters and valve leaflets become smaller and therefore more difficult to repair. Nevertheless, even tibial vein valves have been successfully repaired. If a transposition operation is planned, duplex ultrasound and descending venography must have identified the presence of a competent valve. Valve transplantation requires having identified a valve in the axillary or brachial vein by duplex ultrasonography or venography. Ligation of upper extremity veins is well tolerated. The optimal place for implantation into the lower extremity is into the least damaged vein segment available, which will prevent axial reflux into the calf area.
Prophylactic antibiotics are given during the procedure (e.g., first-generation cephalosporin) but are continued postoperatively for 2 or 3 days by most surgeons.41,44,51 Intraoperative heparin is commonly given when venous occlusion is required during valve repair; a typical dose is 2000 to 10,000 units, which is not generally reversed.38,41,44,51–53
Techniques
Overall Exposure
If reconstruction of the proximal femoral veins (femoral or profunda femoris) is the goal, a groin incision is made in the direction of the vessels to expose the first and second femoral valves.48 Further dissection through the fascia that lies beneath the sartorius muscle will expose the profunda femoris vein if necessary. After exposure of the valve targeted for repair and the adjoining segment of vein, the strip test should be performed to evaluate the competence of the valve This test entails milking blood antegrade past the valve while inflow is occluded, with subsequent application of retrograde pressure against the valve. Reflux is demonstrated by refilling of the vein distally. This incision may also provide exposure for valve transposition or transplantation, if it is required.
After identification of the valve station to be repaired, careful adventitial dissection to identify the valve attachment lines is useful.52 This dissection facilitates proper placement of the venotomy so as not to damage the valve leaflets, when required, and verifies that valve repair is feasible. A lack of valve attachment lines can signify destruction of the valve and suggests the need to consider techniques other than in-situ repair.52
In other situations, a more distal femoral, popliteal, or even tibial vein may be the desired location for valve repair or transplantation. In these situations, the medial exposure is much like that used for exposure of the like-named artery.47 The popliteal vein can be exposed through a posterior S-shaped incision, with the transverse incision made in the posterior knee crease to decrease the chance of scar contraction.47
Internal Valvuloplasty
For decades, the open method of direct valve cusp tightening (reefing) has been the mainstay for correction of primary deep venous valvular reflux. This technique involves venotomy and suturing of the elongated valve leaflets under direct visualization. The redundant valve cusps are plicated to the vein wall with interrupted 6 or 7-0 polypropylene suture to allow proper alignment and later coaptation of the cusps. Kistner in 1968 was the first to report success using a longitudinal venotomy extending through the valve commissure (Fig. 60-4A).54 Raju later described a supracommissural approach in which a transverse venotomy was performed at least 2.5 cm above the valve (Fig. 60-4B).55 Sottiurai described a combination approach in 1988 in which a supravalvular transverse venotomy with distal extension into the valve sinus (a T-shaped venotomy) was used (Fig. 60-4C).56 The “trap door” approach of Tripathi and Ktenidis (2001) involved two transverse incisions on the vein connected by a single vertical incision (Fig. 60-4D).57 Regardless of the approach, suturing of the valve leaflets remains essentially the same (Fig. 60-5). It is estimated that plication of approximately 20% of the length of the valve leaflet can generally restore competency to the valve in the majority of cases.58
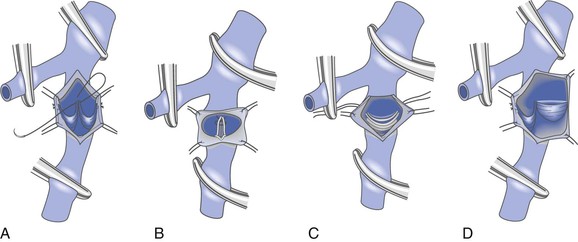
Figure 60-4 Internal valvuloplasty can be accomplished by a variety of techniques used to visualize the incompetent valve leaflets. A, Artist’s representation of the method first proposed by Kistner, in which he opens the vein through the anterior commissure. B, Representation of the method of Raju, who uses a supravalvular transverse venotomy to view the valve from above without incising through the valve commissural angle. C, Representation of the method of Sottiurai, who performs a supracommissural incision with extension toward a cusp sinus to improve visualization. D, Representation of the technique used by Tripathi, who uses a “trap door” incision to provide optimal visualization of the valve cusps for repair. The method of reefing the valve to reestablish a competent valve is essentially the same in each instance.
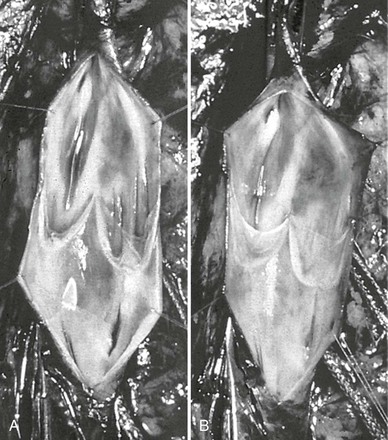
Figure 60-5 The method of Kistner is shown in these operative pictures before valvuloplasty (A) and after valvuloplasty (B). Note the laxity of the incompetent valve before intervention, whereas after reefing of the valve cusps, the normal architecture and tension are reestablished. (Courtesy Dr. Robert Kistner.)
External Valvuloplasty
The technique of external valvuloplasty was pioneered by Kistner and reported in 1990 (Fig. 60-6).59 This approach offers the advantage of valve repair without a venotomy. It is performed by placing interrupted sutures transmurally through the valve attachment lines, which on tying leaves a decreased commissural angle and a competent valve. Others have used a running suture to accomplish a similar effect.52 Both anterior repair and posterior repair can be performed. Another group of investigators combined angioscopy with limited anterior valve sinus plication by interrupted transmural sutures in 15 limbs and achieved improvement in clinical symptoms, hemodynamics, and competence as determined by descending venography.53,60
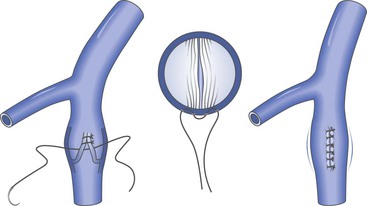
Figure 60-6 Artist’s rendition of the method of external valvuloplasty. The enlarged venous diameter results in valve incompetence. Note how the placement of sutures decreases the diameter of the venous wall but keeps the valve cusps from harm’s way. The sutures lie outside the vein lumen in this approach.
A modification of external valvuloplasty involves the transluminal placement of sutures to reef the valve leaflets and to narrow the vein wall, much like internal valvuloplasty but without opening the vein. Gloviczki and colleagues in 1991 reported using an angioscope to directly view the valve cusps during repair (Fig. 60-7).61 A side branch of the great saphenous vein allows introduction of the angioscope, which is then advanced above the appropriate femoral vein valve for optimal visualization. Interrupted external sutures are seen entering the venous lumen and can be precisely placed to sync up the cusps at the point of attachment on the vein wall. Closure of the valve attachment angle and simultaneous tightening of the valve cusps are realized. Raju contends that use of the angioscope is not necessary after a learning curve but concedes that excellent visualization of the valve is achieved when it is used.52 He believes that identifying the valve cusp externally with the use of adventitial dissection is critical to a technically successful repair and places his sutures as demonstrated in Figure 60-8 (transcommissural valvuloplasty).44 A continuous suture can also be used to accomplish this repair.41 Limited anterior plication, a modification of this method, involves anterior vein dissection and placement of a running mattress suture at the anterior commissure, which runs from a point 3 to 4 mm proximal to the angle of the valve cusp insertion lines up to the angle of the valve cusp insertion.62 About 3 mm of the vein wall is incorporated into the stitch to adequately approximate the cusps.62 Limited anterior plication was developed with the aim of decreasing vein dissection and potentially reducing the progressive dilatation of the postrepair vein that occurs after some cases of valvuloplasty.62 It has been used most commonly in conjunction with saphenous vein stripping and limited to the femoral vein valve station.
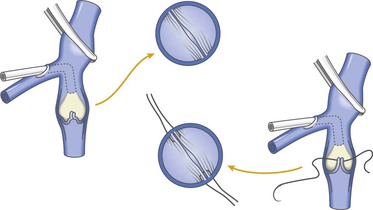
Figure 60-7 As an improvement to “blind” placement of sutures in the method of external valvuloplasty, the use of an angioscope as suggested by Gloviczki and coworkers allows precise suture placement within the vein lumen for actual tightening (reefing) of the valve cusps.
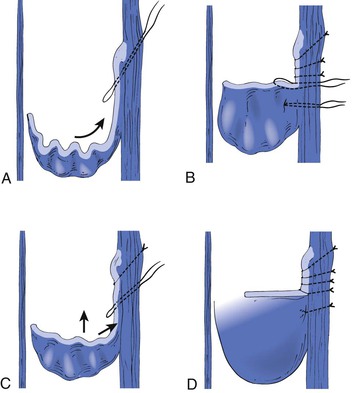
Figure 60-8 The initial through-and-through oblique transluminal suture placed at the commissural apex catches sagging leaflets and resuspends them. A to D, Transluminal sutures, with each successive suture biting deeper and less oblique than the suture above to pull up the valve, tighten the cusp edges, deepen the sinus, and appose the valve attachment lines. Each suture is tied before the next is placed. One or two of the most caudally placed sutures may actually pass through the body of the leaflet rather than through the edge, with no subsequent ill effects. (Redrawn from Raju S, et al: Transcommissural valvuloplasty: technique and results. J Vasc Surg 32:969-976, 2000.)
External Banding
This method has most commonly been used in cases in which dissection vasospasm renders an incompetent valve spontaneously competent. An external sleeve made of polyester (Dacron) or polytetrafluoroethylene is wrapped around the circumference of the vein at the site of the valve. It is tightened to reduce the size of the vein lumen until valve competence is achieved (Fig. 60-9). The sleeve is then anchored in place to the adventitia by sutures to avoid migration. A more aggressive approach of repairing valves that may not spontaneously become competent with simple dissection venoconstriction has been used by one investigator and involves placement of an external valvular stent made specifically for this indication (Venocuff II, Imthage, Sydney, NSW, Australia).51 It is Dacron-reinforced silicone with an adjustable diameter that has been found to be histologically superior to Dacron or polytetrafluoroethylene in animal experimentation. By serially reducing the vein diameter to 30% or less, the valves were rendered competent, but in the rare case when this did not occur, the solution was to go to another valve with the same technique. One report described the use of a spiral device called the Vedensky Spiral that screwed around the outside of the vein to decrease the vein diameter by approximately 30% with endoscopic confirmation of valve competence.63
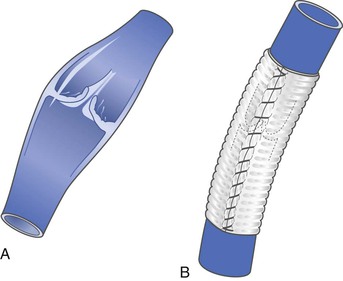
Figure 60-9 Artist’s depiction of external banding to prevent venous valvular incompetence in veins that become competent during the vasoconstriction that occurs at the time of operative dissection. Some surgeons use this technique to prevent dilatation after valve transplantation or other types of repair. A, Incompetent valve before banding. B, After banding, the valve is competent.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree