Chronic Obstructive Pulmonary Disease
GENERAL PRINCIPLES
Definition
• Chronic obstructive pulmonary disease (COPD) is a common, preventable, treatable, and usually progressive condition characterized by persistent airflow limitation (i.e., not fully reversible) and an enhanced chronic inflammatory response to noxious particles or gases.
• Patients with COPD have emphysema and/or airways disease (e.g., chronic bronchitis).
 Emphysema, defined pathologically, consists of nonuniform distal airway enlargement associated with destruction of the acini, loss of lung elasticity, and absence of significant parenchymal fibrosis.
Emphysema, defined pathologically, consists of nonuniform distal airway enlargement associated with destruction of the acini, loss of lung elasticity, and absence of significant parenchymal fibrosis.
 Chronic bronchitis is defined clinically as cough productive of (e.g., at least 2 tablespoons of ) sputum on most days of 3 consecutive months in 2 consecutive years, in the absence of other lung diseases.
Chronic bronchitis is defined clinically as cough productive of (e.g., at least 2 tablespoons of ) sputum on most days of 3 consecutive months in 2 consecutive years, in the absence of other lung diseases.
 COPD has characteristics that overlap with asthma, and both conditions may occur in the same patient (asthma copd overlap syndrome, ACOS).
COPD has characteristics that overlap with asthma, and both conditions may occur in the same patient (asthma copd overlap syndrome, ACOS).
 Although asthma, bronchiectasis, obliterative bronchiolitis, and sarcoidosis often have associated expiratory airflow obstruction, they do not fall within the classification of COPD.
Although asthma, bronchiectasis, obliterative bronchiolitis, and sarcoidosis often have associated expiratory airflow obstruction, they do not fall within the classification of COPD.
Classification
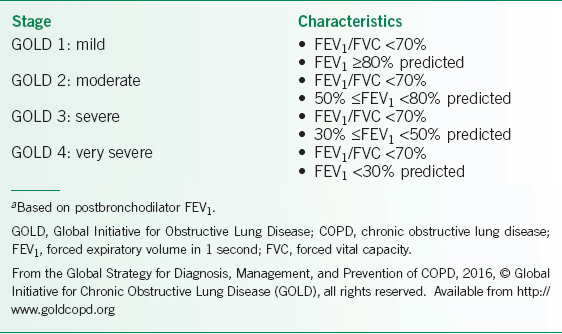
• The Global Obstructive Lung Disease 2015 (GOLD) classification of COPD bases its assessment on the patient’s level of symptoms, exacerbation history, spirometric abnormality, and identification of comorbidities.1
• Postbronchodilator spirometric pulmonary function tests determine the grade of airflow limitation (Table 10-1).1 Height, weight, gender, and sometimes race determine predicted normal values for the forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC).
Epidemiology
• In the United States, ∼5% of the population has COPD.2
• COPD has climbed ahead of stroke to become the third leading cause of death in the United States, following heart disease and cancer.2
Pathophysiology
• Inhaled particles that cause lung inflammation may induce parenchymal tissue destruction (e.g., emphysema) and cause airway disease (e.g., airway fibrosis) through the disruption of normal repair and defense mechanisms.
• Increased mucus production from goblet cell hyperplasia.
• Genetic disorders may create a predisposition to developing COPD.
• Role of airway infections in COPD:
 Defective innate immune responses promote persistent airway bacterial colonization and recurrent airway infections.
Defective innate immune responses promote persistent airway bacterial colonization and recurrent airway infections.
 Acute airway infections often lead to acute exacerbations and subsequently worsened lung function.
Acute airway infections often lead to acute exacerbations and subsequently worsened lung function.
 Viral infections (e.g., influenza, rhinovirus, and adenovirus) and bacterial infection (e.g., Haemophilus influenzae, Streptococcus pneumoniae, Moraxella catarrhalis, and Mycoplasma pneumoniae) cause most exacerbations.
Viral infections (e.g., influenza, rhinovirus, and adenovirus) and bacterial infection (e.g., Haemophilus influenzae, Streptococcus pneumoniae, Moraxella catarrhalis, and Mycoplasma pneumoniae) cause most exacerbations.
TABLE 10-1 2016 GOLD SEVERITY GRADE OF AIRFLOW LIMITATION FOR PATIENTS THAT HAVE COPDA
Risk Factors
• The risk of developing COPD correlates with the total lifetime burden of exposure of inhaled toxins.
 The most important risk factor for the development of COPD is cigarette smoking, which is associated with the majority of cases. However, only a minority of smokers develop clinically significant COPD, suggesting that genetic predisposition and other environmental factors may be required for its development.
The most important risk factor for the development of COPD is cigarette smoking, which is associated with the majority of cases. However, only a minority of smokers develop clinically significant COPD, suggesting that genetic predisposition and other environmental factors may be required for its development.
 Cigar and pipe smokers are also at increased risk of developing COPD.
Cigar and pipe smokers are also at increased risk of developing COPD.
 Occupational exposures and indoor air pollution may lead to COPD.
Occupational exposures and indoor air pollution may lead to COPD.
• Genetic disorders may lead to the development of COPD. α1-Antitrypsin deficiency (A1ATD) contributes to <1% of COPD cases.
 α1-Antitrypsin inhibits neutrophil-derived elastase, an enzyme responsible for the destruction of lung parenchyma in emphysema.
α1-Antitrypsin inhibits neutrophil-derived elastase, an enzyme responsible for the destruction of lung parenchyma in emphysema.
 Patients with A1ATD carry a genetic polymorphism that leads to decreased α1-antitrypsin serum levels.
Patients with A1ATD carry a genetic polymorphism that leads to decreased α1-antitrypsin serum levels.
 A1ATD should be considered in a patient with emphysema who has:
A1ATD should be considered in a patient with emphysema who has:
 COPD and a minimal smoking history.
COPD and a minimal smoking history.
 Early onset COPD (<45 years).1
Early onset COPD (<45 years).1
 Family history of COPD.
Family history of COPD.
 Predominance of lower lobe emphysema seen on imaging studies.1
Predominance of lower lobe emphysema seen on imaging studies.1
Associated Conditions
A number of extrapulmonary comorbidities have been identified in those with COPD such as cardiovascular disease, lung cancer, osteoporosis, skeletal muscle dysfunction, depression, and metabolic syndrome.
DIAGNOSIS
• Symptoms of dyspnea or chronic cough should lead to an evaluation.
• History of heavy smoking should prompt further evaluation in the appropriate clinical setting.
• Spirometry is required to make the diagnosis of COPD.
Clinical Presentation
Symptoms of COPD typically consist of increased dyspnea with exertion, decreased exercise tolerance, and increased sputum production.
History
• Chronic cough and sputum production may precede the development of COPD by many years.
• COPD may develop without chronic cough or sputum production.
• Dyspnea from COPD typically develops after the FEV1 has significantly decreased (e.g., <60% of the predicted normal value) over many years.
• Clinicians should perform a thorough medical history assessment, and question patients regarding symptoms, risk factors, clinical course, comorbidities, medications, and family history.
Physical Examination
Physical examination findings suggestive of COPD do not become apparent until after COPD has significantly progressed, and include:
• Accessory muscle use, pursed lip breathing, and Hoover sign.
• Hyperinflation of the lungs associated with hyperresonant chest percussion.
• Decreased breath and heart sounds.
• Expiratory wheezes variably occur.
• Clubbing of the fingers not expected.
• Symptoms of cor pulmonale occur less commonly:
 Elevated JVP.
Elevated JVP.
 Lower extremity edema.
Lower extremity edema.
 Right ventricular precordial heave, increased S2 and P2 strength, right-sided S3, and tricuspid regurgitation.
Right ventricular precordial heave, increased S2 and P2 strength, right-sided S3, and tricuspid regurgitation.
Diagnostic Criteria
• Spirometry is used to diagnose COPD, and the FEV1 determines the severity of the expiratory airflow obstruction based on the GOLD stages, see Table 10-1 for classification schema.1
• Peak expiratory flow measurement has high sensitivity but low specificity.
• Symptoms and examination findings assist with diagnosis.
• Imaging studies provide evidence of the presence or absence of emphysema.
Differential Diagnosis
• Asthma
• Bronchiectasis
• Reactive airways dysfunction syndrome
• Bronchiolitis obliterans
• Lymphangioleiomyomatosis (LAM)
• Sarcoidosis
• Langerhans cell histiocytosis
• Panbronchiolitis
• Fixed or variable airway obstruction in the upper airways
• Vocal cord dysfunction
• Congestive heart failure (will not cause expiratory airflow obstruction)
• TB
Diagnostic Testing
Laboratory Testing
• We suggest obtaining an arterial blood gas (ABG) if the patient has a low SpO2 (e.g., <92%), FEV1 very low (e.g., <35%), or signs of respiratory or right heart failure occur.
• CBC, to look for polycythemia.
• Complete metabolic panel, to look for elevated bicarbonate level.
• α1-Antitrypsin screening.1
Imaging
CXR posterioranterior and lateral to assess for emphysema or other conditions that could produce similar signs or symptoms.
Diagnostic Procedures
• Pulmonary function tests:
 Spirometry: pre- and postbronchodilator (FEV1/FVC <0.70 and scooping of the expiratory limb of the flow–volume curve).
Spirometry: pre- and postbronchodilator (FEV1/FVC <0.70 and scooping of the expiratory limb of the flow–volume curve).
 Lung volumes (e.g., air trapping [elevated residual volume (RV)] and thoracic hyperinflation [e.g., elevated total lung capacity (TLC)]).
Lung volumes (e.g., air trapping [elevated residual volume (RV)] and thoracic hyperinflation [e.g., elevated total lung capacity (TLC)]).
 Diffusing capacity (e.g., reduced diffusing capacity of the lung for carbon monoxide [DLCO]).
Diffusing capacity (e.g., reduced diffusing capacity of the lung for carbon monoxide [DLCO]).
• Pulse oximetry assessment at rest, with exercise, and possibly during sleep.
• Cardiac testing, when appropriate, to assist with a dyspnea evaluation.
TREATMENT
Acute Exacerbations
• COPD exacerbations are diagnosed clinically based on a worsening in respiratory symptoms beyond the expected day-to-day variation.
• COPD exacerbations typically increase (compared to baseline) one or more of the following:
 Dyspnea
Dyspnea
 Cough and sputum production
Cough and sputum production
 Sputum purulence
Sputum purulence
• The first step taken when encountering a patient with an acute exacerbation should be a quick assessment to determine the need for hospitalization or intensive care unit (ICU) admission for impending respiratory failure (Table 10-2).
• Indications for inpatient admission include
 Marked dyspnea
Marked dyspnea
 New physical findings such as cyanosis or peripheral edema
New physical findings such as cyanosis or peripheral edema
 New or worsened hypoxemia/hypercapnia
New or worsened hypoxemia/hypercapnia
 Lack of adequate response to initial medical management
Lack of adequate response to initial medical management
 Consider hospital admission for those with advanced age or significant comorbidities
Consider hospital admission for those with advanced age or significant comorbidities
• Initial assessment
 CXR
CXR
 ECG
ECG
 ABG, CBC, chemistry panel, brain natriuretic peptide (BNP), and cardiac enzymes.
ABG, CBC, chemistry panel, brain natriuretic peptide (BNP), and cardiac enzymes.
 ABG provides important information about alveolar gas exchange and acid–base status not obtained by pulse oximetry.
ABG provides important information about alveolar gas exchange and acid–base status not obtained by pulse oximetry.
 ABGs can differentiate between acute and chronic respiratory acidosis, and may indicate a need for assisted ventilation and ICU admission.
ABGs can differentiate between acute and chronic respiratory acidosis, and may indicate a need for assisted ventilation and ICU admission.
• Additional testing
 Consider chest CT to evaluate for pulmonary embolism.
Consider chest CT to evaluate for pulmonary embolism.
 Spirometry is not recommended during an exacerbation.
Spirometry is not recommended during an exacerbation.
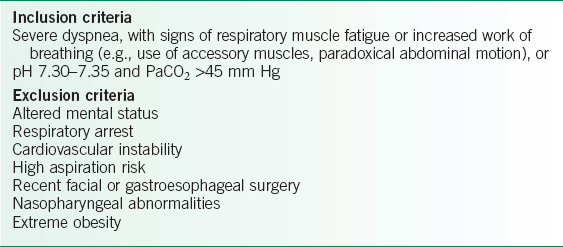
TABLE 10-2 INDICATIONS FOR ICU ADMISSION

Medications
• General considerations regarding bronchodilator therapy
 First-line therapy for symptomatic management of a COPD exacerbation.
First-line therapy for symptomatic management of a COPD exacerbation.
 Multiple randomized controlled trials have demonstrated the similar efficacy of short-acting β2-agonists (SABA) and short-acting anticholinergic (SAAC) agents for rapidly improving symptoms during an acute COPD exacerbation.
Multiple randomized controlled trials have demonstrated the similar efficacy of short-acting β2-agonists (SABA) and short-acting anticholinergic (SAAC) agents for rapidly improving symptoms during an acute COPD exacerbation.
 Combination therapy using a SABA/SAAC has added benefits beyond either agent alone (reduction in hospital length of stay duration and increase in FEV1).
Combination therapy using a SABA/SAAC has added benefits beyond either agent alone (reduction in hospital length of stay duration and increase in FEV1).
 Combination therapy with SABA/SAAC may also have a more rapid onset of action, longer duration of action, and fewer side effects (owing to smaller doses of each individual agent) than use of higher doses of a single agent.
Combination therapy with SABA/SAAC may also have a more rapid onset of action, longer duration of action, and fewer side effects (owing to smaller doses of each individual agent) than use of higher doses of a single agent.
 Long-acting agents are typically not recommended for the management of acute exacerbations of COPD because of the risk of side effects in combination with high-dose short-acting bronchodilator therapy and a lack of demonstrated efficacy and safety in this setting. Long-acting agents should typically be initiated prior to hospital discharge.
Long-acting agents are typically not recommended for the management of acute exacerbations of COPD because of the risk of side effects in combination with high-dose short-acting bronchodilator therapy and a lack of demonstrated efficacy and safety in this setting. Long-acting agents should typically be initiated prior to hospital discharge.
• Inhaled SABA
 Albuterol may be administered q30–60min as tolerated. Subsequent treatment frequency can be decreased, eventually to q4–6h, as the acute exacerbation begins to resolve.
Albuterol may be administered q30–60min as tolerated. Subsequent treatment frequency can be decreased, eventually to q4–6h, as the acute exacerbation begins to resolve.
 β2-agonists may cause tremor, nervousness, tachycardia, tachyarrhythmias, and hypokalemia.
β2-agonists may cause tremor, nervousness, tachycardia, tachyarrhythmias, and hypokalemia.
• Inhaled SAACs
 Ipratropium may be dosed at 4–8 puffs or nebulized q4–6h for a COPD exacerbation.
Ipratropium may be dosed at 4–8 puffs or nebulized q4–6h for a COPD exacerbation.
 Ipratropium is generally well tolerated and tends to produce fewer of the other side effects characteristic of β2-agonist agents.
Ipratropium is generally well tolerated and tends to produce fewer of the other side effects characteristic of β2-agonist agents.
 Anticholinergic agents may cause dry mouth, dry eyes, bladder outlet obstruction/urinary retention, and acute angle glaucoma exacerbation.
Anticholinergic agents may cause dry mouth, dry eyes, bladder outlet obstruction/urinary retention, and acute angle glaucoma exacerbation.
• Corticosteroids
 Systemic administration of corticosteroids is recommended during acute exacerbations of COPD requiring hospitalization.
Systemic administration of corticosteroids is recommended during acute exacerbations of COPD requiring hospitalization.
 Corticosteroids minimize recovery time, decrease hospital length of stay, reduce the incidence of relapse, and improve lung function toward baseline.
Corticosteroids minimize recovery time, decrease hospital length of stay, reduce the incidence of relapse, and improve lung function toward baseline.
 A randomized trial of patients hospitalized for COPD exacerbations found that oral systemic corticosteroids were noninferior to IV corticosteroids.3
A randomized trial of patients hospitalized for COPD exacerbations found that oral systemic corticosteroids were noninferior to IV corticosteroids.3
 The most common adverse effect of systemic corticosteroid administration is hyperglycemia, but other acute adverse effects include but are not limited to systemic hypertension, insomnia, and mood changes.
The most common adverse effect of systemic corticosteroid administration is hyperglycemia, but other acute adverse effects include but are not limited to systemic hypertension, insomnia, and mood changes.
 A typical steroid regimen includes prednisone 30–40 mg PO per day followed by a taper over 10–14 days.
A typical steroid regimen includes prednisone 30–40 mg PO per day followed by a taper over 10–14 days.
 Outpatient management
Outpatient management
 Short courses of oral steroids in patients with moderate to severe COPD can improve the outcomes of patients with exacerbations discharged from the emergency department (ED).
Short courses of oral steroids in patients with moderate to severe COPD can improve the outcomes of patients with exacerbations discharged from the emergency department (ED).
 Inhaled steroids currently do not have a role in the treatment of acute COPD exacerbations.
Inhaled steroids currently do not have a role in the treatment of acute COPD exacerbations.
• Antibiotics
 Current methods do not reliably differentiate bacteria-caused exacerbations from those produced by viruses.
Current methods do not reliably differentiate bacteria-caused exacerbations from those produced by viruses.
 Commonly implicated bacterial pathogens: S. pneumoniae, nontypeable H. influenzae and Haemophilus parainfluenzae, Chlamydia pneumoniae, and M. catarrhalis.
Commonly implicated bacterial pathogens: S. pneumoniae, nontypeable H. influenzae and Haemophilus parainfluenzae, Chlamydia pneumoniae, and M. catarrhalis.
 Sputum cultures in the absence of pneumonia or bronchiectasis are likely of little benefit.
Sputum cultures in the absence of pneumonia or bronchiectasis are likely of little benefit.
 Antibiotics (usually for 5–10 days) are recommended during a COPD exacerbation for those with1:
Antibiotics (usually for 5–10 days) are recommended during a COPD exacerbation for those with1:
 Increased sputum purulence and sputum volume and increased dyspnea OR
Increased sputum purulence and sputum volume and increased dyspnea OR
 Have increased sputum purulence with only one other cardinal symptom OR
Have increased sputum purulence with only one other cardinal symptom OR
 Requiring mechanical ventilation.
Requiring mechanical ventilation.
 Because of rampant antibiotic resistance, particularly in S. pneumoniae, broader-spectrum antibiotic coverage is commonly recommended for acute exacerbations, using one of the following:
Because of rampant antibiotic resistance, particularly in S. pneumoniae, broader-spectrum antibiotic coverage is commonly recommended for acute exacerbations, using one of the following:
 Amoxicillin/clavulanate
Amoxicillin/clavulanate
 Respiratory fluoroquinolone (e.g., levofloxacin or moxifloxacin)
Respiratory fluoroquinolone (e.g., levofloxacin or moxifloxacin)
 Macrolide antibiotic (e.g., azithromycin, erythromycin, or clarithromycin)
Macrolide antibiotic (e.g., azithromycin, erythromycin, or clarithromycin)
• Methylxanthines
 The role of parenteral or oral methylxanthines (e.g., theophylline) during an acute exacerbation is unclear.
The role of parenteral or oral methylxanthines (e.g., theophylline) during an acute exacerbation is unclear.
 Considered third-line agents due to their narrow therapeutic window and potential for severe side effects
Considered third-line agents due to their narrow therapeutic window and potential for severe side effects
Other Nonpharmacologic Therapies
• Oxygen
 Oxygen should be administered to achieve a PaO2 of >55–60 mm Hg (≥89–90% oxyhemoglobin saturation on pulse oximetry).
Oxygen should be administered to achieve a PaO2 of >55–60 mm Hg (≥89–90% oxyhemoglobin saturation on pulse oximetry).
 Worsening hypercapnia may occur with oxygen administration in patients with baseline hypercapnia, and ABG should be checked ∼30–60 minutes after starting oxygen therapy.
Worsening hypercapnia may occur with oxygen administration in patients with baseline hypercapnia, and ABG should be checked ∼30–60 minutes after starting oxygen therapy.
 An increased or new requirement for supplemental oxygen may indicate the presence of a complicating condition such as pulmonary embolism, pneumonia, pneumothorax, or heart failure.
An increased or new requirement for supplemental oxygen may indicate the presence of a complicating condition such as pulmonary embolism, pneumonia, pneumothorax, or heart failure.
• Noninvasive ventilation
 Noninvasive positive-pressure ventilation (NIPPV) is useful for improving oxygenation, decreasing hypercapnia, and relieving work of breathing in patients with acute COPD exacerbation and acute respiratory failure.
Noninvasive positive-pressure ventilation (NIPPV) is useful for improving oxygenation, decreasing hypercapnia, and relieving work of breathing in patients with acute COPD exacerbation and acute respiratory failure.
 NIPPV decreases intubation and mortality rates.4
NIPPV decreases intubation and mortality rates.4
 Methods of NIPPV
Methods of NIPPV
 Continuous positive airway pressure ventilation (CPAP) improves oxygenation and work of breathing, but generally not an underlying acute respiratory acidosis.
Continuous positive airway pressure ventilation (CPAP) improves oxygenation and work of breathing, but generally not an underlying acute respiratory acidosis.
 Bilevel positive airway pressure ventilation (BiPAP) is the preferred method for treatment of respiratory failure associated with acute respiratory acidosis. The larger the gradient between high and low pressure settings, the greater the ventilatory support (improvement in PCO2) provided by NIPPV.
Bilevel positive airway pressure ventilation (BiPAP) is the preferred method for treatment of respiratory failure associated with acute respiratory acidosis. The larger the gradient between high and low pressure settings, the greater the ventilatory support (improvement in PCO2) provided by NIPPV.
 Most patients tolerate NIPPV, which has been show to decrease mortality, the need for endotracheal intubation, and length of hospitalization.
Most patients tolerate NIPPV, which has been show to decrease mortality, the need for endotracheal intubation, and length of hospitalization.
 To be effective, patients must be awake and cooperative with NIPPV. See inclusion and exclusion criteria for NIPPV in Table 10-3.
To be effective, patients must be awake and cooperative with NIPPV. See inclusion and exclusion criteria for NIPPV in Table 10-3.
• Follow-up: For patients hospitalized for a COPD exacerbation, discharge plans should include reinforcement of smoking cessation, review of home medication regimen, assessment of metered dose inhaled (MDI) technique and training when needed, vaccination updates, education, oxygen assessment, and outpatient pulmonary rehabilitation, and healthcare provider follow-up.
TABLE 10-3 CRITERIA FOR NONINVASIVE POSITIVE PRESSURE VENTILATION
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


