and Thomas A. LaMattina2
(1)
Harvard Medical School Cardiology Division, Department of Medicine, Massachusetts General Hospital, Boston, MA, USA
(2)
Harvard Medical School Interventional Cardiology, Cardiology Division, Department of Medicine, Massachusetts General Hospital, Boston, MA, USA
Abstract
Over the last two decades, due to improvement in the management of acute coronary syndromes (ACS), the increasing aging of the population, and the epidemics of diabetes and obesity, the management of patients with chronic coronary artery disease (CAD) has become an increasingly common and important part of clinical practice. The spectrum of chronic ischemic heart disease includes patients who have asymptomatic myocardial ischemia, stable angina pectoris, unstable angina, or prior myocardial infarction (MI) with residual ischemia. Mortality from ischemic heart disease increases in all age groups as blood pressure, vascular stiffness and endothelial dysfunction become more prevalent. New insights into the mechanisms underlying chronic stable coronary disease have led to the emergence of new anti-ischemic treatments and the role for revascularization has been reformulated.
Abbreviations
ACE
Angiotensin converting enzyme
ACS
Acute coronary syndromes
ARB
Angiotensin receptor blockers
CABG
Coronary artery bypass graft
CAD
Coronary artery disease
CCB
Calcium channel blockers
CCS
Canadian Cardiovascular Society
CTA
Computed tomography angiography
EBCT
Electron beam CT
ECG
Electrocardiographic
FFR
Fractional flow reserve
HCTZ
Hydrochlorothiazide
IVUS
Intravascular ultrasound
LAD
Left anterior descending
LBBB
Left bundle branch block
LM
Left main
LV
Left ventricular
MI
Myocardial infarction
MRI
Magnetic resonance imaging
PCI
Percutaneous coronary intervention
SPECT
Single photon emission computed tomography
WPW
Wolff–Parkinson–White
Introduction
Over the last two decades, due to improvement in the management of acute coronary syndromes (ACS), the increasing aging of the population, and the epidemics of diabetes and obesity, the management of patients with chronic coronary artery disease (CAD) has become an increasingly common and important part of clinical practice. The spectrum of chronic ischemic heart disease includes patients who have asymptomatic myocardial ischemia, stable angina pectoris, unstable angina, or prior myocardial infarction (MI) with residual ischemia. Mortality from ischemic heart disease increases in all age groups as blood pressure, vascular stiffness and endothelial dysfunction become more prevalent. New insights into the mechanisms underlying chronic stable coronary disease have led to the emergence of new anti-ischemic treatments and the role for revascularization has been reformulated.
Epidemiology
The prevalence of angina in patients with CAD in epidemiological studies increases with age in both sexes [1].
After development of stable angina, the 2-year incidence of non-fatal MI and cardiovascular death were 14.3 and 5.5 % in men and 6.2 and 3.8 % in women
Prognosis can vary considerably dependent on baseline clinical, functional, and anatomical factors.
Prognostic assessment and risk stratification is an important part of the management of patients with chronic CAD.
It is important to select those patients who could potentially benefit from revascularization, in contrast to those in whom medical therapy may be appropriate.
The presence of conventional cardiovascular risk factors for the development of CAD (hypertension, hypercholesterolemia, diabetes, family history of premature CAD, and smoking) can adversely influence prognosis in patients with established stable CAD.
Treatment aiming on reducing these risk factors can positively influence outcomes in these patients.
Left ventricular (LV) function is the strongest predictor of survival in patients with chronic stable CAD followed by the distribution and severity of coronary artery stenosis.
Pathophysiology
Cardiac ischemia results from an imbalance between myocardial oxygen supply and demand.
Cardiac ischemia can cause either an acute coronary syndrome (ACS) (ST elevation MI, Non-ST elevation MI, or unstable angina) or chronic stable angina.
A sudden reduction in myocardial oxygen supply caused by atherosclerotic plaque injury and thrombosis is usually the mechanism of ACS.
In contrast, an increase in myocardial oxygen demand in the setting of inadequate coronary perfusion and a limited ability to increase myocardial oxygen supply is usually the mechanism of ischemia in chronic stable CAD.
Ischemia-induced sympathetic activation can further increase the severity of myocardial ischemia through a further increase of myocardial oxygen consumption and coronary vasoconstriction.
Natural History
As coronary atherosclerosis progresses, there is deposition of atherosclerotic plaque beneath the intima [4]. Plaque extends eccentrically and outward without significantly compromising luminal diameter (Fig. 4-1).
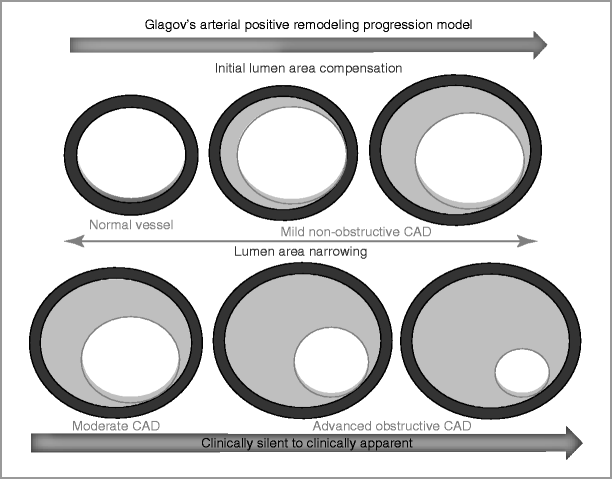
Figure 4-1
Progression of CAD and arterial remodeling (The Glagov’s arterial remodeling model)
In this stage, stress testing or angiography may not identify obstructive CAD even in the presence of significant atherosclerosis.
As atherosclerosis progress, encroachment of the atherosclerotic plaque into the lumen can result in hemodynamic obstruction and angina
Either stress testing or coronary angiography can detect these abnormalities.
In this situation, the functional nature of the obstructive coronary lesion can be also detected by fractional flow reserve measured during cardiac catheterization.
The ischemic cascade is characterized by a sequence of events, resulting in metabolic abnormalities, perfusion mismatch, regional and then global diastolic and systolic dysfunction, electrocardiographic (ECG) changes, and angina.
Adenosine released by ischemic myocardium has been shown to be the main mediator of angina through stimulation of A1 receptors located on cardiac nerve endings.
Myocardial ischemia may also be silent.
In patients who exhibit painless ischemia, dyspnea and palpitation may represent anginal equivalents.
Dyspnea in these cases may also be due to ischemic LV systolic or diastolic dysfunction, or due to ischemic mitral regurgitation.
When luminal obstruction is less than 40 %, maximal flow during exercise can usually be maintained. But luminal diameter reduction of more than 50 % may be associated with ischemia when coronary blood flow becomes inadequate to meet cardiac metabolic demand during exercise or stress.
For a similar degree of stenosis, the ischemic threshold is influenced by other factors including the degree of development of collateral circulation and coronary vascular tone.
Patients with stable angina are at risk of developing an ACS [4].
The hemodynamic severity of the atherosclerotic plaque prior to destabilization is frequently mild, and the plaques are lipid-rich and vulnerable to rupture or erosion [5].
Activation of inflammatory cells within the atherosclerotic plaque appears to play an important role in atherosclerotic plaque destabilization, ultimately leading to plaque erosion, fissure, or rupture.
Clinical Presentation, Diagnosis, and Risk Stratification
Signs and Symptoms
The diagnosis of angina pectoris in patients with chronic coronary disease includes reproducible left-sided anterior chest discomfort triggered by physical activity or emotional stress.
Location, character, duration, and relation to exertion and other exacerbating or relieving factors may define the characteristics of discomfort related to myocardial ischemia.
These symptoms are typically worse in cold weather or after meals and are relieved by rest or sublingual nitroglycerin.
Typical features of stable angina include complete reversibility of the symptoms and repetitiveness of the anginal symptoms over time.
William Heberden first introduced the term ‘angina pectoris’ in 1772 although its pathological etiology was not recognized until years later [8, 9].
Chest pain is characterized as typical angina, atypical angina, and non-cardiac chest pain.
Angina is a syndrome that includes discomfort in the chest, jaw, shoulder, back, epigastric area or arm. The chest pain is aggravated by exertion or emotional stress and relieved by rest and/or nitroglycerin.
Atypical angina is generally defined by two of the above three features, and non-cardiac chest pain is generally defined as chest pain that meets 1 or none of the above criteria (Fig. 4-2).
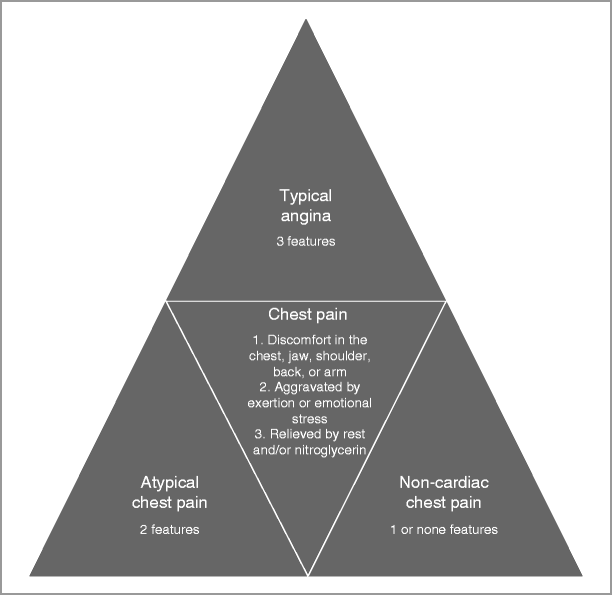
Figure 4-2
Differential diagnosis of chest pain
In order to classify the severity of symptoms based on the threshold at which symptoms occur in relation to physical activities, the Canadian Cardiovascular Society (CCS) Classification (Table 4-1) is an useful tool to determine the functional impairment of the patient and to quantify response to therapy [10].
Table 4-1
Grading of angina pectoris by the Canadian Cardiovascular Society (CCS) Classification System
Class I
Class II
Class III
Class IV
Ordinary physical activity does not cause angina, such as walking, climbing stairs. Angina occurs with strenuous, rapid, or prolonged exertion at work or recreation
Slight limitation of ordinary activity. Angina occurs on walking or climbing stairs rapidly, walking uphill, walking or stair climbing after meals or in cold, or in wind, or under emotional stress. Angina occurs on walking more than two blocks on the level and climbing more than one flight of ordinary stairs at a normal pace and in normal condition
Marked limitations of ordinary physical activity. Angina occurs on walking 1–2 blocks on the level and climbing 1 flight of stairs in normal conditions and at a normal pace
Inability to carry on any physical activity without chest discomfort. Anginal symptoms may be present at rest
Alternative classification systems such as the Seattle angina questionnaire [11] may also be used to determine the functional status of the patient and to quantify response to therapy and may offer superior prognostic information.
Physical signs in patients with angina pectoris are non-specific.
During or immediately after an episode of myocardial ischemia, a third or fourth heart sound may be heard.
A new murmur of mitral regurgitation may be apparent.
Other signs that can be present include xanthelasma in patients with dyslipidemia, lung crackles and elevated jugular venous pressure in patients with heart failure, and other signs of vascular disease such as diminished peripheral pulses and vascular bruits.
Laboratory Testing
Laboratory tests are of low utility in chronic stable angina and therefore are not recommended routinely in patients with chronic coronary disease.
Classical cardiac biochemical markers of myocardial injury such as troponin or CKMB are usually negative, however highly-sensitive troponins may be elevated in those with stable coronary disease.
Natriuretic peptides such as BNP or NT-proBNP may be mildly elevated in those with stable angina.
Fasting plasma glucose [12, 13] and lipid profile [14] should be evaluated in all patients with suspected coronary disease to establish the patient’s cardiovascular risk profile.
Renal function should be initially evaluated in patients with chronic coronary disease.
Renal dysfunction may occur due to associated vascular comorbidities and has a negative impact on prognosis in patients with CAD [15].
Additional laboratory testing, including cholesterol subfractions (ApoA and ApoB) [16], homocysteine [17], lipoprotein (a) (Lpa), coagulation profile, NT-proBNP [18], and markers of inflammation (hs-C-reactive protein) [19, 20], have been used to improve risk prediction in selected patients, but presently lack a defined therapeutic response if found to be elevated.
Non-Invasive Diagnostic Testing
Resting electrocardiogram
All patients with suspected stable coronary artery disease should have a resting 12-lead ECG recorded.
A normal or non-specific ECG is not uncommon and does not exclude the diagnosis of chronic myocardial ischemia.
Patients with normal baseline ECG have better prognosis since that usually implies normal LV function.
The resting ECG may show signs of CAD such as previous MI or an abnormal ST-T segment with ST depression and/or T wave inversion.
Other ECG findings may include left ventricular hypertrophy, bundle branch blocks, AV node block, atrial fibrillation, and frequent ventricular ectopy, all which can predict worse prognosis in patients with CAD.
ECG stress testing
Exercise ECG is more sensitive and specific than the resting ECG for detecting myocardial ischemia and is the test of choice to identify inducible ischemia in patients with suspected stable angina.
Horizontal or down-sloping ST segment depression of more than 1 mm defines a positive test with a sensitivity and specificity for the detection of significant coronary disease of 68 and 77 %, respectively [21].
Exercise ECG testing is not of diagnostic value in the presence of left bundle branch block (LBBB), paced rhythm, and Wolff–Parkinson–White (WPW) syndrome, in which cases, the ECG changes cannot be evaluated. Other confounders are the use of digoxin, resting ECG depression greater than 1 mm, and the presence of LV hypertrophy.
ECG changes associated with myocardial ischemia when accompanied by chest pain suggestive of angina during exercise, especially when these changes occur at a low workload and persist during the recovery period further increases the specificity of the test.
A fall in systolic blood pressure or lack of increase of blood pressure during exercise, the appearance of a systolic murmur of mitral regurgitation, or the presence of ventricular arrhythmias during exercise may reflect more severe CAD and increase the probability of severe myocardial ischemia.
The Duke treadmill score is a well-validated score that combines exercise time, ST-deviation, and angina during exercise to calculate the patient’s risk (Fig. 4-3) [22, 23].
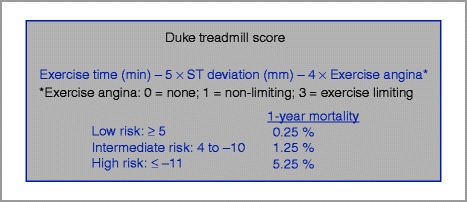
Figure 4-3
Duke Treadmill Score
Interpretation of stress testing requires a Bayesian approach (Fig. 4-4).
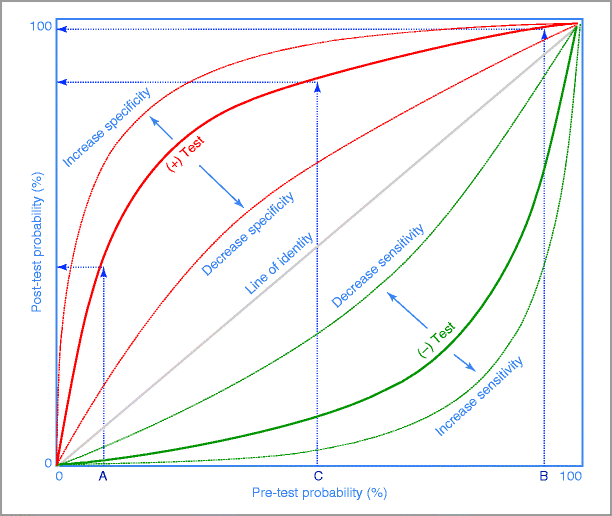
Figure 4-4
Bayes’ Theorem diagram. The post-test probability of disease after a test is influenced not only by the sensitivity and specificity of the test but also by the pre-test probability of disease. In patients with a low pre-test probability of disease (A), a positive test result will minimally increase the post-test probability and therefore have a low discrimination power. Similarly, in patients with high pre-test probability (B), a positive test will only confirm the presence of disease and therefore have a low discrimination power. The higher discrimination power of the test occurs in patients with intermediate pre-test probability of disease (C). For a given pre-test probability, the post-test probability becomes progressively higher as the test becomes more abnormal. As the sensitivity of the test increases, the negative test curve shifts further away from the line of identity. As the specificity of the test increases, the positive test curve shifts away from the line of identity
This dictates that the post-test probability of a true positive result is based on the pre-test probability of disease presence.
Diagnosis using the Bayes’ formula is a probabilistic assessment and not a binary decision (true or false).
Once the presence of symptoms that may represent obstructive CAD is identified, the pretest probability of CAD should be assessed. The pre-test probability can be determined by an established risk score, such as the Framingham Risk Score and further modified by the nature of symptoms at an individual patient level.
Diamond and Forrester described the relationship between clinical symptoms and angiographically significant CAD (Table 4-2) [24].
Table 4-2
Pre-test probability of CAD by age, gender, and symptoms
Age (years)
Gender
Typical angina
Atypical chest pain
Non-cardiac chest pain
Asymptomatic
30–39
Men
Intermediate
Intermediate
Low
Very low
Women
Intermediate
Low
Very low
Very low
40–49
Men
High
Intermediate
Intermediate
Low
Women
Intermediate
Low
Very low
Very low
50–59
Men
High
Intermediate
Intermediate
Low
Women
Intermediate
Intermediate
Low
Very low
60–69
Men
High
Intermediate
Intermediate
Low
Women
High
Intermediate
Intermediate
Low
Patients with symptoms of angina and intermediate pre-test probability of coronary disease based on age, gender, and symptoms, unless unable to exercise or have ECG changes that make ECG non-evaluable are candidates for ECG stress testing as the initial assessment tool for diagnosis and risk stratification.
Imaging stress testingGet Clinical Tree app for offline access
Stress imaging offers superior diagnostic performance over conventional exercise ECG testing for the detection of obstructive CAD.
The ability to quantify and localize areas of ischemia makes stress-imaging techniques often preferred in patients with previous revascularization.
Myocardial perfusion scintigraphy using 201Th and 99mTc radiopharmaceuticals are the most commonly used methods of imaging employing single photon emission computed tomography (SPECT) in association with an exercise test on either a stationary bicycle or a treadmill.
Reported sensitivities and specificities are approximately 90 and 90 % respectively.
The SPECT myocardial perfusion imaging Appropriateness Criteria recommends:
For patients without symptoms:
Use of radionuclide stress imaging only in high cardiovascular risk patients.
In those asymptomatic patients with intermediate cardiovascular risk with a non-interpretable ECG, the use of radionuclide imaging was considered of uncertain benefit.
In symptomatic patients:
Radionuclide imaging is considered appropriate if patients had an intermediate or high likelihood for CAD by pre-test assessment. Imaging test was also considered appropriate for symptomatic patients at low likelihood of CAD if they were unable to exercise or had a non-interpretable ECG.< div class='tao-gold-member'>Only gold members can continue reading. Log In or Register to continue

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


