Clinical findings
CBD
Sarcoidosis
Onset
Insidious
Acute or insidious
Restrictive lung disease
Yes
Yes
Obstructive lung disease
Frequent
Yes
Reduced diffusion capacity
Yes
Yes
Erythema nodosum
No
Yes
Lupus pernio
No
Yes
Neurologic manifestations
No
Yes
Bone cysts
No
Yes
Extrapulmonary manifestations without pulmonary involvement
No
Yes
Ophthalmologic manifestation
Conjunctivitis only
Conjunctivitis, uveitis, retinal involvement
Hepatic manifestations
Occasional
Common
Cardiac manifestations
Rare
Occasional
Hypercalcemia
Rare
Rare
Chest imaging
Isolated hilar or mediatinal adenopathy
Very rare
Common
Parenchymal ground glass opacities
Common
Common
Parenchymal nodules
Yes
Yes
Bronchial stenosis
Yes
Very rare
Subpleural cysts
Yes
Rare
Conglomerate masses
Yes
Rare
Laboratory findings
Beryllium sensitization
Yes
No
Radiographic appearance of CBD on chest X-ray or CT-scan is identical to that of sarcoidosis, although mediastinal or hilar lymphadenopathy is less common. Chest radiographs range from small nodular opacities, with an upper level predominance, to formation of conglomerate masses or can be normal. Moreover, even HRCT and pulmonary function tests can be normal in patients with granulomatous lung disease and therefore the diagnosis CBD must not be excluded on the basis of those negative results [32]. Mediastinal and hilar lymphadenopathy are present in approximately a third of individuals examined by chest radiograph or computed tomography. Further radiographic features are listed in Table 30.1. In aggregate, there are no radiographic findings differentiating CBD from sarcoidosis.
Beryllium sensitization is the first immunologic event leading to CBD but it does not result in any physical impairment. A clear exposure dose dependency could be established [24]. At present there is no medical therapy to prevent progression to CBD. However, theoretical considerations and epidemiological studies suggest that termination of exposure may remit sensitization [33]. Further exposure and its cumulative dose define progression to CBD [24] which can take place after a short time or after a latency of years or decades. Precipitating cofactors are not known [19, 33]. Overall, under continued exposure a progression rate of 6–8 % per year of sensitized is reported [19]. After manifestation of CBD many patients suffer from slow progression of symptoms and defects of pulmonary function which can precede radiographic abnormalities [34]. However, next to those protracted courses rapid ones are observed [31]. According to CBD registry data and epidemiological studies from the United States of America, mortality rates of CBD patients vary widely from 6 to 38 %. In addition to CBD excess mortality rates for chronic obstructive pulmonary disease, lung cancer, urinary tract cancer, and nervous system cancer are reported [33, 35, 36]. Whether advances in diagnosing early disease and consecutive termination of exposure have lowered this number seems likely, however, is not known.
Diagnosis and Differential Diagnosis
The diagnosis of berylliosis is an epicritical one made when a case of non-necrotizing granulomatous disease – otherwise diagnosed as sarcoidosis – is accompanied by documented occupational (or in rare cases ambient) beryllium-exposure in combination with beryllium-hypersensitivity. Granulomatous disease of other origin such as bacterial, fungal, viral, helminthic, or metallic need to be excluded in a diagnostic workup and idiopathic disorders such as idiopathic pulmonary fibrosis may mimic CBD. The primary condition to be ruled out is sarcoidosis and the features listed in Table 30.1 are of some help. Certain clinical findings are more characteristic for sarcoidosis, such as extensive hilar adenopathy in the absence of parenchymal infiltrates (radiographic Type I of sarcoidosis) and spontaneous resolution, or have never or only rarely been reported in berylliosis, such as cystic bone lesions and cranial or peripheral nerve involvement. None of these clinical features, however, is adequately sensitive or specific to reliably distinguish between sarcoidosis and berylliosis in individual patients. Other disorders to be considered in differential diagnosis are listed in Table 30.2.
Table 30.2
Granulomatous disorders to be considered in the diagnostic workup in suspected chronic beryllium disease
Cause of granuloma formation | Disease |
|---|---|
Bacterial | Brucellosis, Bartonella henselae (cat scratch fever), chlamydia (Lymphgranuloma venereum), leprosy, salmonellosis, tuberculosis |
Fungal | Blastomycosis, coccidiomycosis, histoplasmosis |
Viral | Measles virus |
Helminthic | Filariasis, schistosomiasis, trichinosis |
Metallic | Aluminium-, beryllium-, titanium-, zirconium- induced lung disease |
Bioaerosols | Hypersensitivity pneumonitis (Rectivirgula faeni, Trichosporon cutaneum) |
Drugs | Allopurinol alveolitis |
Unknown | Crohn’s disease, granulomatous polyangitis, sarcoidosis |
Box 30.1
Proposed Criteria for Working Diagnosis
Chronic beryllium disease
A granulomatous disorder otherwise diagnosed as sarcoidosis needs to be established.
Evidence of exposure.
Proof of beryllium sensitization by positive Be-LPT findings or a positive equivalent.
Indium Tin Oxide-Lung Disease
The diagnosis requires a compatible clinical appearance combined with an occupational exposure. Clinical clues for ITO-lung disease can be:
Reticular nodular shadows with fibrosis and cholesterol clefts +/− emphysematous lesions
Pulmonary alveolar proteinosis without or only moderate concentration of GM-CSF autoantibodies.
Hard metal induced lung disease
evidence of diffuse parenchymal lung disease by HRCT,
evidence of pulmonary function defects and
histological examination of lung specimens demonstrating giant cell interstitial pneumonitis.
Flock worker’s lung
persistent respiratory symptoms;
previous work in the flocking industry;
histologic evidence of interstitial lung disease compatible with flock worker’s disease
Asbestosis
evidence of diffuse parenchymal lung disease either by HRCT or histology,
evidence of a causal relationship by demonstrating environmental history of asbestos exposure with plausible latency,
markers of exposure such as pleural plaques or recovery of asbestos bodies (BAL, lung specimen)
exclusion of competing diagnoses.
Nanoparticle induced lung disease:
exposure to nanoparticles at the workplace,
evidence of a diffuse parenchymal lung disease by HRCT,
evidence of pulmonary function defects and
histological examination of lung specimens demonstrating interstitial lung disease and nanoparticles.
A pivotal step in the diagnosis of CBD is the demonstration of beryllium sensitivity by beryllium-lymphocyte proliferation test (Be-LPT). At present Be-LPT with blood or bronchoalveolar lavage mononuclear cells is the only routine laboratory test available to prove beryllium hypersensitivity [37]. Skin tests such as patch test or intracutaneous tests should be avoided because there is a considerable risk of inducing sensitization by intra cutaneous application of beryllium salts and clear diagnostic readout criteria are not defined. Originally bronchoalveolar lavage cells have been employed in the Be-LPT [37] but for practicability reasons it has been adopted for the use of peripheral blood mononuclear cells [38]. Blood Be-LPT is used for diagnostic workup and occupational monitoring. Since Be-LPT is a biological ex-vivo test requiring viable cells usual quality standards of the clinical laboratory cannot be applied. The test should be performed in a laboratory with experience in cell biological testing and in some countries this test might not be available on a routine basis. In the United States of America laboratories offering Be-LPT are accredited according to the Clinical Laboratory Improvement Amendments. Similar procedures have to be established in most other countries.
To perform Be-LPT mononuclear cells have to be isolated from bronchoalveolar lavage or anti-coagulated (EDTA, heparin) peripheral venous blood. The latter one can be sent by overnight express to the laboratory but special containers and shipping conditions need to be employed which have to be tuned with the laboratory. For Be-LPT with bronchoalveolar lavage cells lavage needs to be performed at the site of the laboratory and to obtain sufficient numbers of cells larger volumes of 200 ml and more need to be installed. Cell culture without any additive is performed to estimate background proliferation and by addition of mitogens the viability of the cells is demonstrated. The upper limit of background proliferation is laboratory dependent and needs to be individually established using mononuclear cells of unexposed controls. The mean plus two standard deviations is usually taken for the upper limit but other limits are also in use. To obtain a reliable value multiple cell cultures should be performed. In the authors laboratory 16 parallel cultures are used to establish background proliferation of patient and control cells. Cell cultures with different concentrations of BeSO4 over at least a three log-range between 10−4 and 10−6 mol/L are run in quadruplicate or more and proliferation is estimated once or several times between 3 and 8 days of culture. Tests with at least two elevated proliferation values are considered abnormal. Mitogen-induced proliferation serves as non-specific control [38, 39]. It has to be noted that outside the United States of America this test is not standardized and some laboratories use absolute numbers of stimulation indices as threshold or request dose-dependent proliferation for positive tests. As a consequence the clinician has to familiarize with the generation of the thresholds in the employed laboratory. Cell culture details of the test should be reported by the laboratory for correct interpretation of results. Since a specific positive control is not available, some authors demand two independent tests to accept the clinical consequence [40]. A test specification released by the Department of Energy of the United States of America in 2001 (Specification 1142–2001) to standardize Be-LPT for epidemiological purposes can be used as guideline to evaluate or to establish the test. Examples of positive and negative results from the laboratory of the authors are shown in Fig. 30.1.
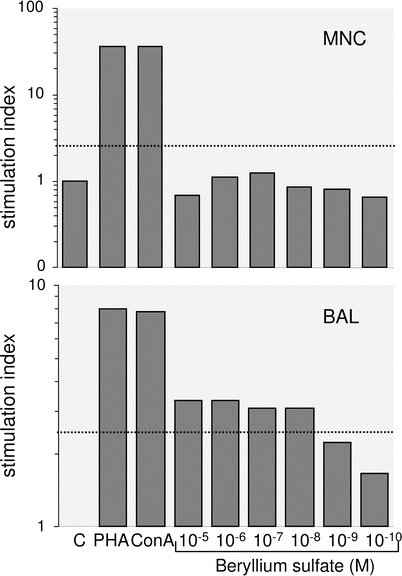

Fig. 30.1
Beryllium-lymphocyte proliferation tests with peripheral blood mononuclear cells (MNC, top) and bronchoalveolar lavage cells (BAL, bottom) of a patient with chronic beryllium disease are shown demonstrating the higher sensitivity of BeLPT using BAL-cells. The test with MNCs reveals a negative and the one with BAL cells a positive result. C: stimulation index (SI) of non-stimulated cells yields the background DNA replication and is set 1.0, PHA, ConA (phytohemagglutinin, concanavalin A): stimulation with lectins causes a high SI demonstrating the viability of the cells in in-vitro culture. From the variation of the SI in C individual thresholds are calculated which are indicated by horizontal dotted lines. MNCs exhibit SIs below the threshold in all beryllium sulfate concentrations but cultures with BAL cells disclose SIs above the threshold in 4 out of 6 concentrations indicating the sensitization of this patient. The figure depicts the means of octuplet cultures for every concentration
It has to be noted that the sensitivity of Be-LPT from peripheral blood is under debate. Reported sensitivities comparing multiple testings to identify false negatives range between 38 % [39] and 100 % [38] with low interlaboratory reproducibility [41]. Consequently, there are cases of berylliosis which have not been diagnosed due to false negative test results. Thus, in those cases with negative Be-LPT results and doubtless exposure, the tentative diagnosis of CBD has to be either excluded or verified with multiple independent tests. The high specificity of Be-LPT, however, is generally accepted since positive test results have not been reported in non-exposed controls or patients suffering from other granulomatous disorders [38, 39, 42]. Its positive predictive value is comparable to other accepted medical tests with a sensitivity of 0.683, a specificity of 0.969, and a positive predictive value of one abnormal test of 0.253 [40]. In the case of any doubt the test can be repeated with cells from bronchoalveolar lavage which has been demonstrated to be more sensitive due to an higher proliferation capacity of these cells in response to beryllium [37] (see Fig. 30.1). However, this can be markedly reduced by the effect of cigarette smoking causing a predominance of macrophages in the recovered cells potentially masking the lymphocyte response. Immunosuppresive compounds including steroids may dampen proliferation in response to beryllium and cause false negative results of Be-LPT. Although not systematically studied, most centers recommend discontinuation of steroid treatment for at least 3 weeks up to 3 months before Be-LPT is performed. However, own experience demonstrates that abnormal Be-LPTs can be obtained under low dose steroid treatment.
Not every individual with a positive Be-LPT suffers from CBD [43]. Some beryllium exposed individuals have repeatedly positive results demonstrating sensitization without pulmonary granulomas or other signs of disease. In a follow-up study 31 % of those individuals progressed to symptomatic CBD within 4 years [19]. At present the test still remains the standard diagnostic test, since it is the only way to add an etiologic criterion. Whether patients with a positive Be-LPT in a surveillance program and the demonstration of granuloma suffer from early disease when they are asymptomatic and do not develop pulmonary function defects is a matter of debate.
The use of Be-LPT is cumbersome and alternatives using different readouts of cell activation are under investigation. Flow cytometry [44, 45], ELI-spot techniques [4, 46] and other cytokine based assays may be close to clinical practice. The use of metabolomic signatures to identify CBD and to differentiate CBD from beryllium sensitization is still in its infancy [47].
Measuring beryllium in urine and tissue samples may unequivocally identify exposure, however, concentrations of biological relevance are far below the sensitivity of routine tests which limits the clinical value of negative results [25, 48–50].
Thus, for the unequivocal diagnosis of CBD the following criteria should be fulfilled:
A granulomatous disorder otherwise diagnosed as sarcoidosis needs to be established.
Evidence of exposure.
Proof of beryllium sensitization by positive Be-LPT findings or a positive equivalent.
In this context it has to be noted that granulomatous disease is not regarded mandatory for making the diagnosis of CBD. Mononuclear alveolitis in the presence of beryllium exposure and hypersensitivity in combination with symptomatic disease may be sufficient to support the diagnosis. This is why some authors suggest for reasons of practicability to omit histopathologic criteria [30]. Many different criteria are used to define chronic beryllium disease. The differences in these criteria reflect the change in our understanding of the disease pathophysiology and the availability of diagnostic tests. Other differences may be related to the purpose of making the diagnosis such as clinical care, surveillance, research, or compensation.
Exposure
Beryllium-exposed individuals may be unaware of their exposure and physicians may be unaware of beryllium-related health effects leading to non-recognized beryllium sensitization and CBD. Therefore, an occupational case history covering the entire professional life is mandatory in the diagnostic workup of granulomatous disorders. Because CBD manifestation can take place a long time after exposure has ceased this should preferably be done with the help of an expert in occupational medicine which is, however, not practicable in most cases. Thus, a basic knowledge of beryllium usage is helpful to select patients to be referred to occupational medicine. Short or paraoccupational exposures may be missed without expert awareness of hazardous workplaces. Therefore, basic information on beryllium properties and uses in industries and technical trades is given in the following.
Beryllium is a metallic element found in beryl and bertrandite ores and processed into beryllium oxide, beryllium metal, beryllium alloys and composite materials. The mineral beryl is a beryllium aluminium cyclosilicate with the chemical formula Be3Al2(SiO3)6. Pure crystals of beryl are colorless and vary in size. Tinted with chrome they are green emeralds and tinted with iron or titan they are blue aquamarines. More than 80 % of the world’s beryllium ore mining and processing is done in the United States. Kazakhstan and China are also beryllium producers. The most important product is copper alloy containing 0.15–2.0 % beryllium.
Copper-beryllium alloys withstand high temperatures and mechanical stress. They are extraordinarily hard but flexible, resistant to corrosion, do not spark, and are nonmagnetic. Beryllium and its alloys share a number of properties with aluminum, which explains their frequent use in aerospace and defense industry. Because the addition of beryllium improves the electrical and thermal conductivity of alloys, this metal is also frequently used in electronic and microelectronic applications such as semiconductor devices and integrated circuits requiring heat dissipation. Springs, switches, relays, and connectors in computers, radar, automobiles, telecommunication equipment, tools, and other instruments contain beryllium. Copper-beryllium is a common substrate for gold plated electrical connectors. Copper-beryllium scrap is often mingled with copper scrap for recycling. As a result, workers in both the metal recycling and precious metal recovery industries encounter beryllium.
Beryllium is used in casting of many different alloys where it refines the grain size resulting in better surface polishing, reduces melt losses, and improves casting fluidity. It also finds use as an acid catalyst in organic reactions, and as an additive to glass and plastics. Neutron moderators or reflectors in nuclear reactors and X-ray windows also contain beryllium. It is frequently found in gems and, depending on work processes, gem polishers are exposed. Jewelers may be exposed when precious stones are framed or polished. Optical crystals also contain beryllium; as a result beryllium-exposure takes place in the production of precision optical instruments including fiberoptics.
Beryllium oxide is the most important high-purity commercial beryllium chemical produced and its primary use is in the manufacturing of ceramics. Because beryllium-oxide is transparent to microwaves, it is also used in microwave devices.
Thus, the workers potentially exposed to beryllium are beryllium ore miners, beryllium alloy fabricators, phosphor manufacturers, ceramic workers, missile technicians, nuclear reactor workers, electric, electronic, and optical equipment workers, and jewelers. Noteworthy, workers in down-stream industries and crafts using beryllium-containing parts may be exposed. Past exposure of workers involved in fluorescent powder manufacture and in the manufacture and salvage of fluorescent lamps may still cause disease. The recycling of electronic parts is a relatively new workplace with implied beryllium exposure. Work places and their products with potential exposure are listed in Table 30.3.
Table 30.3
Workplaces, components, and products with potential beryllium-exposure
Additives to glass, ceramic, plastics | Golf clubs | Pen clips |
Aerospace industries (e.g. aircraft frames, engines, and brakes) | Gyroscopes | Personal computers |
Automobile industries (engines, electronic parts) | Metallurgic industries/recycling | Precision instruments |
Brass alloys | Microelectronics | Recycling workplaces |
Camera shutters | Microwave devices | Satellites |
Ceramic industries | Military vehicle armor | Springs |
Chemical industries | Mirrors | Structural material in space technology |
Dental workshops | Missile production and maintenance | Submarine cable housings |
Electrical relays | Missile guidance systems | Transistor mountings |
Electronic industries | Nonsparking tools | Wheels |
Fluorescent lamp production/disposal | Nuclear reactors and industries | X-ray tubes |
Gems | Optical industries/workshops |
Treatment and Monitoring
The first therapeutic measure is elimination of exposure and occupational studies reported reversibility of physiologic and radiographic defects when exposure is reduced or terminated [51, 52]. Although there are no studies demonstrating unequivocally a benefit of this step, it is recommended for CBD patients. Whether its social implications are justified in sensitized individuals has to be decided on an individual basis in combination with a genetic counseling [53]. Patients with early disease (i.e. sensitization in combination with granuloma but without symptoms or lung function defects) should be monitored using routine lung function tests, exercise physiology, and chest radiographs to detect progressive disease which is considered an indication for corticosteroid therapy. Serological markers of disease activity used in sarcoidosis, such as angiotensin converting enzyme, soluble interleukin-2 receptor or neopterin, can be used to gauge the inflammatory activity of CBD [54–56]. However, treatment decisions need to be made on the basis of symptoms and progressing organ dysfunction.
Systemic corticosteroids are the mainstay of CBD treatment and drug regimens established for sarcoidosis are used. Starting doses of 0.5–0.8 mg prednisolone per kg body weight per day are recommended, and stabilization or improvement will take place in most patients. However, under tapering the dose or after cessation of therapy some patients relapse, which may result in long-lasting maintenance therapy. The response to corticosteroids in CBD is quite variable. Long lasting remissions and recalcitrant disease have been observed [57]. Frequently recalcitrant disease can be suppressed with low dose corticosteroid maintenance therapy [57]. There have been no systematic studies of the use of other immunosuppressive, immunomodulatory or anti-inflammatory drugs in CBD. For patients who either do not respond to high doses of prednisolone, or require unacceptable high maintenance doses, second line therapy should be guided by experience in sarcoidosis, and corticosteroid-sparing regimens can be recommended as a second step [58, 59]. Relatively few patients progress to end-stage lung disease and lung transplantation should be offered to those who qualify for this type of therapy.
Supportive and rehabilitative therapy should be used as necessary. These include supplemental oxygen if rest or exercise-induced hypoxemia is present, bronchodilators if bronchial hyperresponsiveness or obstructive lung disease is present, pulmonary rehabilitation to maintain muscle strength and tone.
Prevention of Beryllium Sensitization and Chronic Beryllium Disease
After diagnosing CBD competent authorities have to be informed to take action for the prevention of other workers at this particular workplace, their family members, and habitants in the neighborhood of the workplace. A Be-LPT screening program may be appropriate to identify beryllium sensitization and latent CBD in those cohorts although the high variability of Be-LPT makes its use difficult in cohorts with low prevalence [60]. However, with careful epidemiologic guidance this type of program can yield clinical and occupational important results but several positive tests might be required for a definite diagnosis [40, 61, 62].
Although genetic factors determining susceptibility for beryllium sensitization and the risk for progression to CBD are known [14] a genetic counseling cannot be suggested in primary or secondary prevention because the expected postintervention CBD prevalence rates might not be low enough in the light of serious ethical, social, or legal concerns [53]. The hypersensitivity nature of the CBD implies that a complete eradication by industrial hygiene measures will not be possible as long as the use of beryllium is maintained. However, primary prevention by mandatory exclusion of individuals testing positive for certain genetic markers from workplaces with potential beryllium-exposure is no practical approach since the predictive value of the known markers is too low to enable an ethically correct verdict [53]. Voluntary genetic counseling of sensitized workers may be a cost-effective way of preventing CBD, however sufficient data to do so is only available for the Caucasian ethnicity and therefore ethical and legal implications may prevent implementation [14, 53].
Indium Tin Oxide-Lung Disease
Indium–tin oxide (ITO) is a sintered alloy containing a large portion (≈90 %) of indium oxide and a small portion (≈10 %) of tin oxide. It is used in the production of thin-film transistor liquid crystal displays (LCDs) for flat-panel displays used in television screens, touch screens, solar cells, and architectural glass. The use of ITO containing compounds in the electronics and semiconductor industry has risen by 500 % over the last two decades. Little is known about the potential health hazard induced by occupational exposure to indium compounds. However, pulmonary toxicity has been demonstrated in experiments with hamsters.
In 2003 the first case of ITO interstitial pneumonia was identified by demonstrating indium and tin in intraalveolar particles by energy dispersive X-ray analysis of a patient suffering from interstitial lung disease. Physical examination disclosed clubbing and fine crackles with high pitched squeaks on auscultation. Chest CT-scan showed ground glass opacities all over the lung and subpleural honeycombing. Exposure time was 3 years but exposure dose could not be estimated. Therapy with prednisolone was initiated but no improvement was observed. The patient died from bilateral pneumothorax 7 years after first exposure [63]. More cases with interstitial pneumonitis, pulmonary fibrosis, and emphysematous defects have been reported in smoking and non-smoking workers from Japan and a causal dose-dependent relationships between ITO-exposure and interstitial and/or emphysematous defects in CT-scans and serum level of KL-6, SP-A and SP-D could be established in a large cross-sectional study with 592 ITO exposed workers in Japan [64, 65] and in 170 workers in Taiwan [66].
Two cases of pulmonary alveolar proteinosis (PAP), including one death, were observed in workers at a facility in the United States of America producing ITO. In one antibodies against granulocyte-macrophage colony stimulating factor (GM-CSF) could be identified suggesting an immunologic mechanism induced by ITO [67]. However, anti GM-CSF could not be found in 17 ITO workers in Japan but reevaluation of Japanese cases demonstrated next to cholesterol clefts periodic acid Schiff (PAS)-positive material in the alveolar space of 4 out of 7 cases [68]. PAS material could also be generated by toxic effects of ITO on alveolar macrophages as shown in an animal study [69]. In aggregate, the observations are compatible with PAP and PAS-positive material in the alveolar space as an acute ITO response which is replaced by cholesterol clefts and fibrosis in the long term [68].
In a study of 108 male ITO workers in the facility in which the first case of ITO-lung disease was observed in 23 workers disclosed significant reticulonodular shadows on HRCT of the chest. In addition, in 14 of those 23 workers emphysematous changes could be seen. These radiographic changes correlated with both the serological marker of alveolitis KL-6 and the length of exposure [65].
As for CBD a suspicion of ITO-lung disease can only arise when a compatible clinical appearance combines with an occupational exposure. Clinical clues for ITO-lung disease can be:
Reticular nodular shadows with fibrosis and cholesterol clefts ± emphysematous lesions
Pulmonary alveolar proteinosis without or only moderate concentration of GM-CSF autoantibodies.
Generally accepted therapeutic recommendations do not exist. Terminating exposure has lead to complete recovery [70]. The value of therapeutic bronchoalveolar lavage or corticosteroid therapy needs to be established [67].
CBD is an exposure-related form of sarcoidosis and alike ITO-lung disease might turn out to be an exposure-related form of PAP or pulmonary fibrosis with emphysema. The relevance of anti-GM-CSF antibodies and elevated serum markers, as KL-6, SP-A or SP-D, in pathogenesis and disease managing has still to be established.
Hard Metal Lung
The term “hard metal” must not be confused with “heavy metals” such as lead, cadmium, and mercury. Hard metal consists to 90–94 % of a tungsten carbide structure which is blended with 6–10 % cobalt as a binder and compressed into a polycrystalline material [71, 72]. It is heat and corrosion resistant and has an extraordinarily mechanical strength almost that of diamond. It is used in tools for drilling, cutting, or grinding [71–73]. Workers exposed to hard metals are toolmakers, blacksmiths, diamond polisher and workers processing steel alloys containing hard metal [71, 74]. Abraham and colleagues were the first to publish that many cases described by Liebow as giant cell interstitial pneumonitis (GIP) were related to hard metal exposure [75]. Later, Ohori and colleagues confirmed the finding that GIP is almost pathognomonic for hard metal or cobalt exposure [76]. However, not every case of hard metal lung disease appears as GIP. Other types of interstitial pneumonia were also documented to be manifestations of hard metal lung disease such as DIP, UIP and BOOP [76]. The presence of multinucleated giant cells can be easily documented by BAL, which can also be used for mineral analysis and detection of tungsten.
Animal experiments and case reports suggest that cobalt is the key agent inducing ILD by hard metal [77–80]. However, Lison and colleagues reported that cobalt bound in hard metal is more toxic than in other compounds indicating that cobalt alone cannot be responsible for the toxicity of hard metal particles [81].
In our outpatient clinic we established the diagnosis of giant cell interstitial pneumonia (GIP) in an 82 year old, never-smoking, retired lady. HRCT revealed subpleural honeycombing combined with diffuse ground glass lesions (Fig. 30.2). She worked for 20 years in a spinning mill and was exposed to cobalt containing paints which lead to the diagnosis of occupational hard metal lung was made. An example of giant cell pneumonitis is shown in Fig. 30.3.
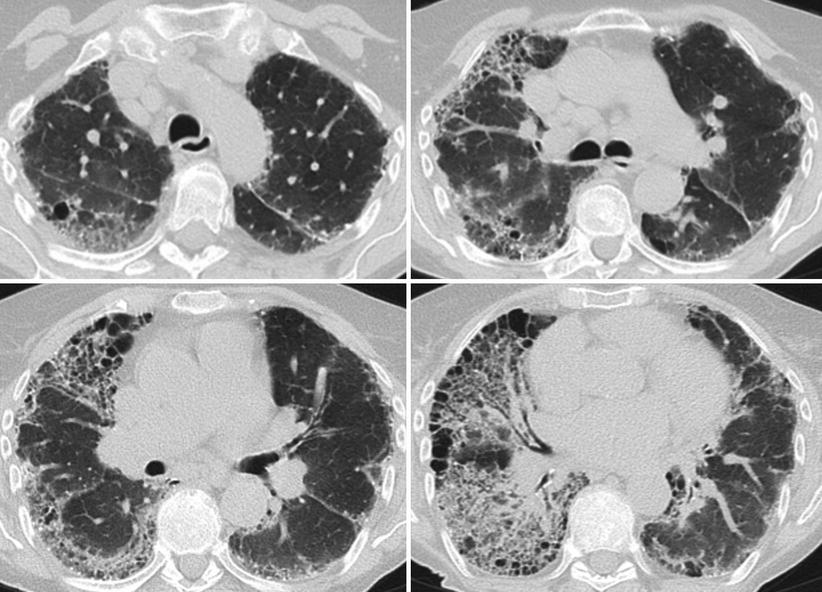
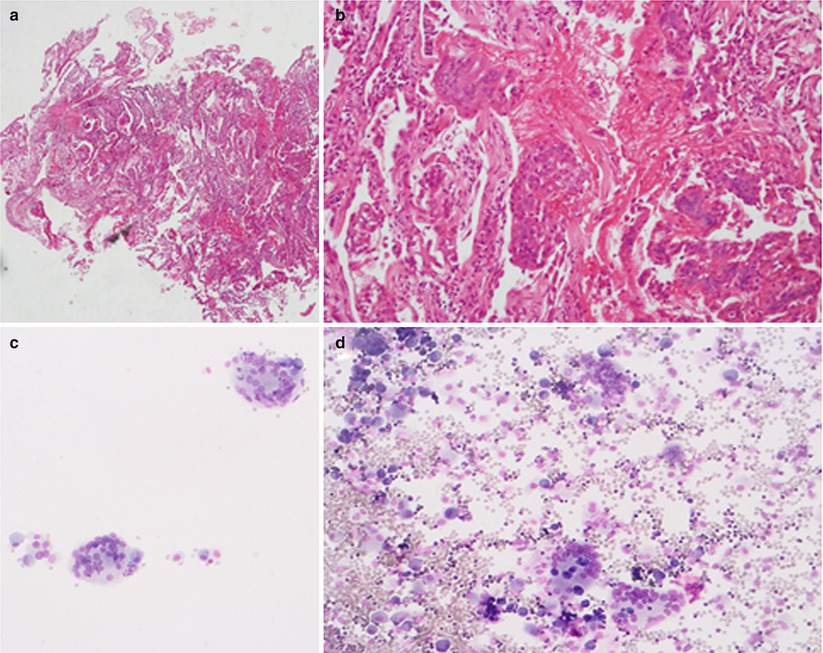

Fig. 30.2
HRCT scan of a 82 year old patient with giant cell interstitial pneumonitis after Cobalt exposure at yarn factory. Histological diagnosis was obtained by transbronchial biopsy and confirmed by wedge biopsy. HRCT shows diffuse severe lesions, predominately in the right lung. There is severe subpleural honeycombing on both sides. Beside honeycombing there are ground glass lesions at the left lung. Right lung shows coarse reticular lesions in the lower central areas and ground glass lesions in the upper regions

Fig. 30.3
Depicted are a transbronchial biopsy and a BAL cytology of a 82 year old lady with giant cell interstitial pneumonitis. Panels a and b: transbronchial biopsy and panels c and d: BAL both with giant cells
In contrast to pneumoconiosis, a dose-response relationship is not evident in hard metal lung disease. Evidence suggests a delayed-type hypersensitivity immune response to be involved and clinical characteristics are often similar to hypersensitivity pneumonitis [82, 83]. Thus, even minimal exposure can cause hard metal lung disease. Course of the disease is variable: some patients might recover completely after avoiding further exposure, while others progress to irreversible pulmonary fibrosis. Older patients tend to have chronic and progressive disease. Several authors reported that patients benefit from prednisolone and other immunosuppressive treatment, but multicenter, placebo-controlled studies are lacking [74, 82, 84, 85].
Unfortunately, criteria for the diagnosis of hard metal induced lung disease which are generally agreed on are missing. Thus, in view of the literature [86, 87] the following criteria are suggested:
evidence of a diffuse parenchymal lung disease by HRCT,
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


