, Marc J. Semigran2 and Marc J. Semigran3
(1)
Harvard Medical School Cardiology Division, Department of Medicine, Massachusetts General Hospital, Boston, MA, USA
(2)
Harvard Medical School, Boston, USA
(3)
Heart Failure and Cardiac Transplant Program, Cardiology Division, Department of Medicine, Massachusetts General Hospital, Boston, MA, USA
Abstract
Chronic heart failure (HF) is a common manifestation of cardiovascular (CV) disease, affecting more than six million adults in the United States. Despite overall improvements in cardiovascular health, the incidence of HF has remained stable due to the aging of the population as well as improved survival following myocardial infarction (MI). Among patients over the age of 65, the incidence of HF is approximately 1 % annually. Major advances have occurred in the understanding of HF pathophysiology and treatment, leading to significant declines in HF-related mortality. However, it remains a cause of significant morbidity and mortality, resulting in more than one million hospitalizations and 50,000 deaths annually.
Abbreviations
ACE
Angiotensin-converting enzyme
ACEI
Angiotensin-converting enzyme inhibitor
AF
Atrial fibrillation
AHA
American Heart Association
ARB
Angiotensin-receptor blocker
BB
Beta-blocker
BMI
Body mass index
BNP
B-type natriuretic peptide
BTT
Bridge to transplantation
CAD
Coronary artery disease
cAMP
Cyclic adenosine monophosphate
CO
Cardiac output
CPET
Cardiopulmonary exercise test
Cr
Creatinine
CV
Cardiovascular
DT
Destination Therapy
HCM
Hypertrophic cardiomyopathy
HDZ
Hydralazine
HF
Heart failure
HFpEF
Heart failure with preserved ejection fraction
HFrEF
Heart failure with reduced ejection fraction
HTN
Hypertension
IABP
Intra-aortic balloon pump
ICD
Implantable cardiac defibrillator
ISDN
Isosorbide dinitrate
JVP
Jugular venous pressure
K
Potassium
LV
Left ventricular
LVAD
Left ventricular assist device
LVEF
Left ventricular ejection fraction
LVH
Left ventricular hypertrophy
MCS
Mechanical circulatory support
MI
Myocardial infarction
NIDCM
Nonischemic dilated cardiomyopathy
NYHA
New York Heart Association
PH
Pulmonary hypertension
PVR
Pulmonary vascular resistance
RHC
Right heart catheterization
RRR
Relative risk reduction
RV
Right ventricular
RVEF
Right ventricular ejection fraction
SCD
Sudden cardiac death
TPG
Transpulmonary gradient
TTE
Transthoracic echocardiogram
VO2
Peak oxygen consumption
WU
Woods Units
Introduction
Chronic heart failure (HF) is a common manifestation of cardiovascular (CV) disease, affecting more than six million adults in the United States [1]. Despite overall improvements in cardiovascular health, the incidence of HF has remained stable due to the aging of the population as well as improved survival following myocardial infarction (MI). Among patients over the age of 65, the incidence of HF is approximately 1 % annually [1]. Major advances have occurred in the understanding of HF pathophysiology and treatment, leading to significant declines in HF-related mortality [2]. However, it remains a cause of significant morbidity and mortality, resulting in more than one million hospitalizations and 50,000 deaths annually [1].
Definition of HF
Inability of heart to pump enough blood to meet metabolic needs of tissues
Can be caused by inability of ventricle to fill, inability to pump or systemic process causing excess metabolic demand
Symptoms: poor exercise tolerance (fatigue or dyspnea)
Signs: evidence of fluid retention or poor perfusion
American Heart Association (AHA) Classification – based on disease progression and therapeutic strategy [3]
Stage A – patients at high risk for development of HF without evidence of structural heart disease or symptoms of HF
Stage B – patients with structural heart disease but without symptoms or signs of HF
Stage C – patients with structural heart disease and current or prior symptoms of HF
Stage D – patients with refractory HF requiring advanced therapies
New York Heart Association (NYHA) classification – based on symptoms
Class I – No limitation of ordinary physical activity
Class II – mild symptoms with ordinary physical activity
Class III – marked limitation of physical activity
Class IV – symptoms of HF at rest or with minimal physical activity
Causes of Chronic HF
Ischemic cardiomyopathy – coronary artery disease (CAD) most common cause of left ventricular (LV) systolic dysfunction in developed countries
MI → regional scar and loss of contractility → adverse remodeling of remaining segments → LV dilatation and dysfunction
If hibernating myocardium present, revascularization may improve LV function
Nonischemic dilated cardiomyopathy (NIDCM)
Idiopathic – up to 50 % of NIDCM [4]
Toxins – HF potentially reversible with removal of offending agent
Alcohol – direct toxic effect on cardiomyocytes
Cocaine – unclear pathophysiology, may include coronary vasospasm, direct myocardial toxicity
Medications (Anthracyclines, Trastuzumab, Cyclophosphamide)
Hypertension (HTN)
Initially causes concentric LV hypertrophy (LVH) but can eventually progress to dilated cardiomyopathy
Viral myocarditis
Myocardial injury caused by virus or autoimmune response to viral remnants
Initial infection may present acutely or may be silent
Other infectious causes
HIV – associated with high viral titers
Chagas Disease – prevalent in Central and South America
Lyme Disease – typically associated with conduction disturbances
Genetic
Familial dilated cardiomyopathy
LV non-compaction
Arrhythmogenic right ventricular cardiomyopathy
Tachycardia-induced cardiomyopathy
Can be due to atrial arrhythmias, ventricular arrhythmias (VAs) or premature ventricular contractions
Resolves with control of heart rate or elimination of arrhythmia
Peripartum Cardiomyopathy
Occurs in last month of pregnancy or within 5 months of delivery
LV function usually improves but high rate of recurrent LV dysfunction with subsequent pregnancies
Hypothyroidism
Obstructive sleep apnea
Uremia
HF with preserved Ejection Fraction (HFpEF)
LV ejection fraction (LVEF) >50 % in approximately half of all HF patients [5]
Compared to patients with LV systolic dysfunction, HFpEF patients are more likely to be older, female, hypertensive, and have atrial fibrillation (AF) [6]
Similar survival to HF with reduced ejection fraction (HFrEF) – median survival of 2.1 years from diagnosis [5, 6]
Presence of diastolic dysfunction (by echo or invasive hemodynamics) required for diagnosis but pathophysiology complex
No treatments proven to prolong survival or decrease HF hospitalizations in HFpEF patients
Valvular heart disease
Any valvular lesion can cause HF symptoms in presence or absence of LV systolic dysfunction
Hypertrophic cardiomyopathy (HCM)
Inherited disorder associated with mutation of sarcomere genes
Present in 1/500 people with varying degrees of expression [7]
Results in marked ventricular hypertrophy, often asymmetric with predominant interventricular septal thickening
May also have mid-ventricular and apical hypertrophy variants
HF symptoms result from dynamic outflow tract obstruction, mitral regurgitation, diastolic dysfunction
First-line therapy are beta-blockers or calcium-channel blockers to reduce contractility and obstruction
Refractory symptoms may respond to surgical septal myectomy or alcohol septal ablation
Restrictive cardiomyopathy
Idiopathic restrictive cardiomyopathy
Infiltrative diseases
Sarcoidosis – usually presents with arrhythmias or sudden death
Amyloidosis – senile, familial, or associated with abnormal light chain production (AL amyloidosis)
Initially normal LV systolic function, with subsequent deterioration of LVEF
Storage diseases
Fabry’s Disease
X-linked genetic disorder
Deficiency of α-galactosidase A → lysosomal storage disease
Characterized by marked LVH – may be confused for HCM
Can be treated with enzyme replacement
Hemochromatosis
Inherited genetic order or secondary to large volume of blood transfusions
Characterized by myocardial deposition of iron
Endomyocardial fibrosis
Diffuse fibrosis of ventricular endocardium of unclear etiology
Most common worldwide cause of restrictive cardiomyopathy
Mostly found in Africa, Asia and South America
Radiation Therapy
Damages blood vessels → inflammation → myocardial fibrosis → decreased ventricular compliance
Right ventricular (RV) failure
Almost always associated with pulmonary hypertension (PH)
Final consequence of many congenital heart lesions, particularly in context of Eisenmenger syndrome (irreversible PH)
Constrictive Pericarditis
May be caused by:
Prior cardiac surgery
Radiation
Infections (Tuberculosis, bacterial, parasitic)
Resolves with surgical pericardiectomy
Pathophysiology of Chronic HF (Fig. 15-1)
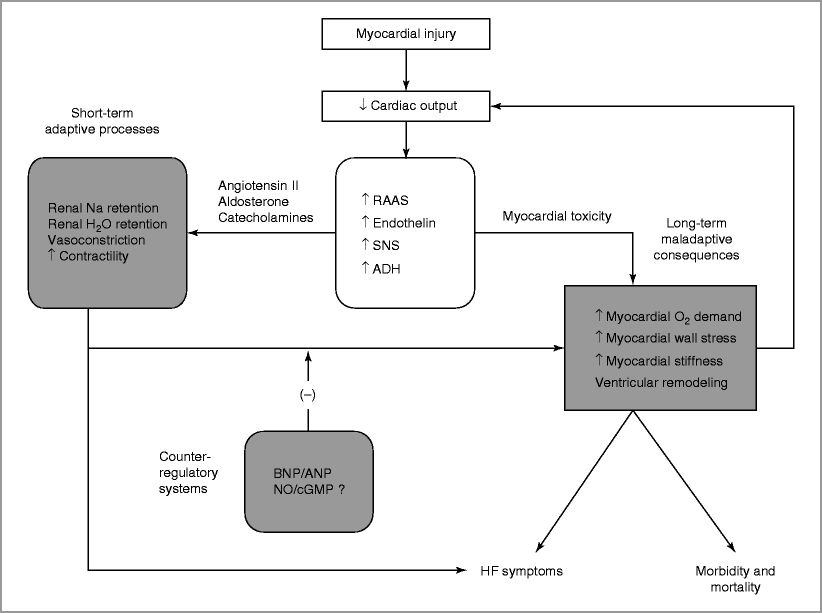
Figure 15-1
A schematic representation of the pathophysiology of chronic heart failure due to impaired LV systolic function. The initiating event is an injury that leads to myocardial dysfunction. The body compensates for decreased cardiac output by activating multiple neurohormonal systems. In the acute phase, these mechanisms act to maintain adequate perfusion of systemic organs, but may also result in congestion and HF symptoms. Over time, these compensatory systems have adverse effects on the LV, stimulating further neurohormonal activation, worsening HF symptoms and ultimately leading to HF mortality. Counter-regulatory systems, including the natriuretic peptides, are upregulated to prevent the adverse effects of neurohormonal activation. Abbreviations: RAAS renin-angiotensin-aldosterone system, SNS sympathetic nervous system, ADH anti-diuretic hormone, BNP B-type natriuretic peptide, ANP atrial natriuretic peptide, NO nitric oxide, cGMP cyclic guanosine monophosphate, HF heart failure
Acute injury to myocardium causes decreased cardiac output (CO) and end-organ perfusion
Neurohormonal activation
Upregulation of renin–angiotensin–aldosterone system
Increased angiotensin II → systemic and renal arterial vasoconstriction
Increased aldosterone → renal sodium retention
Sympathetic nervous system activation
Release of catecholamines (e.g. norepinephrine)
Results in enhanced myocardial contractility and systemic vasoconstriction
Decreases distal water delivery in kidney due to reduction in glomerular filtration rate (GFR) → decreased excretion of water
Release of anti–diuretic hormone
Enhances reabsorption of water by renal collecting tubules
Ventricular remodeling
Type of remodeling depends on type of stress placed on ventricle
Pressure overload (e.g. aortic stenosis)
Concentric remodeling → LVH
Reduced wall stress via LaPlace’s Law (stress inversely proportional to wall thickness)
Volume overload (e.g. mitral regurgitation)
Eccentric remodeling → ventricular dilatation
Increased preload maintains cardiac output via Frank-Starling mechanism
Myocardial injury (e.g. MI)
Stretching of scarred tissue → mixed pressure and volume load on non-infarcted tissue
Ventricular dilatation → maintenance of cardiac output
Acute compensatory responses become deleterious over time
Progressive ventricular dilatation → increased wall stress (LaPlace: stress proportional to chamber radius)
Ongoing ventricular remodeling causes progressive HF
Evaluation of Chronic HF
Comprehensive history with focus on potential etiologies of cardiomyopathy
Detailed alcohol, drug, toxin exposure
Atherosclerotic risk factors, history of MI
Systemic systems indicative of extracardiac disease
Family history of HF, CAD or sudden cardiac death
Symptom assessment
Dyspnea most common symptom
NYHA Classification
Congestion (orthopnea, paroxysmal nocturnal dyspnea)
Low CO (fatigue, impaired cognition)
Physical Examination
Signs of congestion
Rales and/or pleural effusions on pulmonary exam – can be absent in long-standing HF despite elevated left-sided pressures
Elevated jugular venous pressure (JVP)
Positive hepatojugular reflex – sustained rise in JVP with compression of right upper quadrant of abdomen
Ascites
Lower extremity edema
Signs of low CO
Hypotension
Sinus tachycardia
Narrow pulse pressure
Cool extremities
Diminished pulses
Other findings
Displaced and enlarged point of maximal impulse
Third heart sound (S3)
RV heave
Prominent pulmonic component of second heart sound (P2)
Murmurs of functional mitral and tricuspid regurgitation
Diagnostic testing
Laboratory analyses
Basic metabolic panel, complete blood count, liver function tests, thyroid-stimulating hormone, urinalysis, hemoglobin A1C or fasting glucose
HIV test, iron studies (to screen for hemochromatosis) and sleep study should be considered in most patients
Further testing in selected patients depending on risk factors for specific etiologies of HF
ECG – arrhythmias, conduction disturbances, voltage (high or low), ectopy
Chest X-ray – cardiac chamber enlargement, pleural effusions, interstitial or pulmonary edema
Transthoracic echocardiogram (TTE)
LV and RV systolic function
Presence of scar or wall motion abnormalities – suggestive of CAD
Diastolic function of LV
Quantification of chamber dilation and ventricular hypertrophy
Identification of valvular abnormalities
Presence of pericardial effusion
Assessment for obstructive CAD with coronary angiography or noninvasive imaging in patients with CAD risk factors
Cardiopulmonary exercise testing (CPET) – measurement of peak oxygen uptake (VO2) provides assessment of relative contributions of cardiac disease and pulmonary disease to dyspnea as well as prognostic information
Endomyocardial biopsy not helpful in most cases unless specific diagnosis suspected that would alter management
Signal-averaged electrocardiogram not recommended in routine assessment
Prognosis of Chronic HF
Factors associated with worse prognosis in chronic HF include:
LVEF – 39 % increase in mortality for each 10 % drop in LVEF [8]
RV ejection fraction (RVEF) <35 % [9]
PH [10]
QRS length >120 ms [11]
VO2 <14 ml/kg/min [12]
Chronic kidney disease – patients with severe renal dysfunction have 2× risk of death at 1 year compared to patients with normal renal function [13]
B-type Natriuretic Peptide (BNP)/amino-terminal (NT)-proBNP – one of strongest independent predictors of prognosis [14]
Troponin levels [15]
Risk scores have been developed for patient stratification
Seattle Heart Failure Model
Incorporates clinical variables, medications and devices
Highly accurate prediction of survival out to 3 years in general HF population [16]
Heart Failure Survival Score [17]
Incorporates CAD, heart rate, LVEF, blood pressure, intraventricular conduction delay, serum sodium, peak VO2
Used to risk stratify NYHA Class III–IV patients being considered for transplantation
Management of Chronic HF
Treatment of comorbid conditions [3]Get Clinical Tree app for offline access
HTN – Maintenance of blood pressure within guideline-recommended limits
Identification and treatment of dyslipidemia based on risk level
Diabetes – Control of blood glucose within guideline-recommended limits
AF
Control of ventricular rate
Consideration of rhythm control
No survival benefit has been demonstrated but may improve symptoms
Amiodarone and dofetilide only agents with established safety in HF< div class='tao-gold-member'>Only gold members can continue reading. Log In or Register to continue

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


