Chest Wall Deformities
Robert C. Shamberger
A broad spectrum of congenital chest wall deformities occurs. The severe life-threatening deformities, ectopia cordis and asphyxiating thoracic dystrophy, are rare in comparison with the more frequent and milder pectus excavatum and carinatum. Congenital anterior thoracic deformities can be conveniently considered in five categories: (a) pectus excavatum; (b) pectus carinatum; (c) Poland’s syndrome; (d) sternal defects including ectopia cordis; and (e) miscellaneous conditions, including vertebral and rib anomalies, asphyxiating thoracic dystrophy (Jeune’s disease), and rib dysplasia.
Pectus Excavatum
Posterior depression of the sternum and costal cartilages produces the characteristic findings of pectus excavatum: funnel chest, or trichterbrust. The first and second ribs and the manubrium are usually in their normal position (Fig. 43-1), but the lower costal cartilages and the body of the sternum are depressed. In older adolescents and adults, the most anterior portion of the osseous ribs may also be curved posteriorly. The extent of sternal and cartilaginous deformity is quite variable. Numerous methods of grading and defining these deformities have been proposed by Hümmer and Willital,42 Oelsnitz,68 Welch,104 and Haller and associates,33 as well as others, but none has been universally accepted. Asymmetry of the depression is frequently present. Often the right side is more depressed than the left, and the sternum may be rotated as well. Lawson and coauthors52 have recently described a system to quantify the asymmetry based on computed tomography (CT) of the chest. Many children with pectus excavatum have a characteristic physique with a broad thin chest, dorsal lordosis, “hook shoulder” deformity, costal flaring, and poor posture.
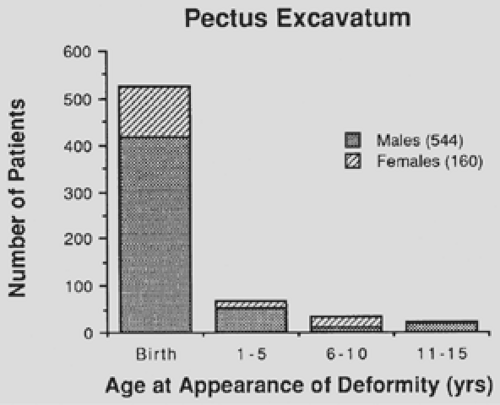
Pectus excavatum is present at birth or within the first year of life in the majority of affected children (86%), as shown in Figure 43-2. The deformity rarely resolves with increasing age and may worsen during the period of rapid adolescent growth. Waters and associates99 identified scoliosis in 26% of 508 patients with pectus excavatum. Hence all patients with pectus deformities should be evaluated clinically for scoliosis. Asymmetric pectus excavatum with a deep right gutter and sternal rotation often is accompanied by scoliosis. Correction of the associated pectus excavatum may stabilize the curve in conjunction with exercises or bracing, thereby avoiding spinal fusion.
Congenital heart disease was identified by Shamberger and Welch89 in 1.5% of infants and children undergoing chest wall correction at the Boston Children’s Hospital (Table 43-1). The frequency of chest wall deformities among all patients with congenital heart disease evaluated at this institution was only 0.17%.
Asthma may be identified in patients with pectus excavatum and carinatum. In a review of 694 consecutive cases, Shamberger and Welch87 found a subgroup of 35 patients with asthma (5.2%), which is comparable with the occurrence of asthma in the general pediatric population.
Etiology and Incidence
Ravitch75 reported that pectus excavatum may occur as frequently as 1 in 300 to 400 live births and that it is rare in blacks.
It occurs more frequently in boys than girls, by almost a 4:1 ratio. Although the sternal depression appears to be caused by overgrowth of costal cartilages, the etiology of pectus deformities is unknown. Early investigators, such as Lester,54 attributed its development to an abnormality of the diaphragm. Little evidence has supported this theory other than the occurrence reported by Greig and Azmy30 of pectus excavatum in children after repair of agenesis of the diaphragm. Vanamo and associates96 also demonstrated a frequent association between congenital diaphragmatic hernia and pectus excavatum. Hecker and associates38 described histopathologic changes in the costal cartilages similar to those seen in scoliosis, aseptic osteonecrosis, and inflammatory processes, but the etiology of these findings and their significance are unknown. An increased familial incidence exists. In a review by Shamberger and colleagues,87 37% of 704 patients had a family history of chest wall deformity. Analysis of 34 families with more than one family member with pectus excavatum by Creswick and coworkers17 has shown a variable pattern of inheritance which appears to be multifactorial.
It occurs more frequently in boys than girls, by almost a 4:1 ratio. Although the sternal depression appears to be caused by overgrowth of costal cartilages, the etiology of pectus deformities is unknown. Early investigators, such as Lester,54 attributed its development to an abnormality of the diaphragm. Little evidence has supported this theory other than the occurrence reported by Greig and Azmy30 of pectus excavatum in children after repair of agenesis of the diaphragm. Vanamo and associates96 also demonstrated a frequent association between congenital diaphragmatic hernia and pectus excavatum. Hecker and associates38 described histopathologic changes in the costal cartilages similar to those seen in scoliosis, aseptic osteonecrosis, and inflammatory processes, but the etiology of these findings and their significance are unknown. An increased familial incidence exists. In a review by Shamberger and colleagues,87 37% of 704 patients had a family history of chest wall deformity. Analysis of 34 families with more than one family member with pectus excavatum by Creswick and coworkers17 has shown a variable pattern of inheritance which appears to be multifactorial.
 Figure 43-1. An 8-year-old boy with asymmetric pectus excavatum deformity. Note that the depression extends to the sternal notch. |
Table 43-1 Cases of Congenital Heart Disease Associated with Pectus Excavatum and Carinatum | ||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||
Three of four siblings were involved in one family. Scherer and colleagues83 reported a high incidence of chest wall deformities in children with Marfan’s syndrome that are often severe and usually accompanied by scoliosis. Patients with abdominal musculature deficiency syndrome (prune-belly syndrome) commonly have pectus excavatum (8 of 43 patients in the experience of Welch and Kearney).105 Pectus excavatum also occurs with other myopathies and chromosomal defects, such as Turner’s syndrome. A summary of the associated musculoskeletal abnormalities is shown in Table 43-2.
Symptoms
Pectus excavatum is well tolerated in infancy and childhood. The anterior depression in an infant with a flexible chest may be magnified by upper airway obstruction from tonsillar and adenoidal hypertrophy, but they do not primarily produce the pectus deformity. Older children may complain of pain in the area of the deformed cartilages or of precordial pain after sustained exercise. A few patients have palpitations, presumably due to transient atrial arrhythmias. These patients may have mitral valve prolapse and associated atrial arrhythmias.
Pathophysiology
Some researchers, including Haller and associates,32 contend that no cardiovascular or pulmonary impairment results from
pectus excavatum. This contrasts, however, with the clinical impression that many patients have increased stamina after surgical repair. These findings date back to the surgical repair performed by Sauerbruch in 1913.81 The patient was an 18-year-old boy who developed dyspnea and palpitations with very limited exercise. Three years after his operation, he could work 12 to 14 hours a day without tiring and without palpitations. Anecdotal reports during the next three decades confirmed this observation. Since then, investigators have sought to identify the physiologic abnormality or combination of abnormalities that could explain this symptomatic improvement after surgery. Early physiologic measurements of cardiac and pulmonary function were crude and did not yield convincing evidence of a cardiopulmonary deficit. In many early studies, summarized by Shamberger and Welch,86 the results fell within the broad range of normal values, if often at the lower limit. In a recent analysis of a large cohort of 408 patients with pectus excavatum, Lawson and coworkers50 found that the median values for forced vital capacity (FVC) and forced expired volume in 1 second (FEV1) were 13% below predicted values and forced expiratory flow (FEF25%–75%) median was 20% below the predicted value.
pectus excavatum. This contrasts, however, with the clinical impression that many patients have increased stamina after surgical repair. These findings date back to the surgical repair performed by Sauerbruch in 1913.81 The patient was an 18-year-old boy who developed dyspnea and palpitations with very limited exercise. Three years after his operation, he could work 12 to 14 hours a day without tiring and without palpitations. Anecdotal reports during the next three decades confirmed this observation. Since then, investigators have sought to identify the physiologic abnormality or combination of abnormalities that could explain this symptomatic improvement after surgery. Early physiologic measurements of cardiac and pulmonary function were crude and did not yield convincing evidence of a cardiopulmonary deficit. In many early studies, summarized by Shamberger and Welch,86 the results fell within the broad range of normal values, if often at the lower limit. In a recent analysis of a large cohort of 408 patients with pectus excavatum, Lawson and coworkers50 found that the median values for forced vital capacity (FVC) and forced expired volume in 1 second (FEV1) were 13% below predicted values and forced expiratory flow (FEF25%–75%) median was 20% below the predicted value.
Table 43-2 Musculoskeletal Abnormalities Identified in 130 of 704 Cases of Pectus Excavatum | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||
A systolic ejection murmur is frequently identified in patients with pectus excavatum and is magnified by a short interval of exercise. It is attributed to the close proximity between the sternum and the pulmonary artery, which results in transmission of a flow murmur.
Electrocardiographic abnormalities are common; Schaub and Wegmann82 attributed them to the abnormal configuration of the chest wall and the displacement and rotation of the heart into the left thoracic cavity. Preoperative electrocardiographic findings reported by Welch104 in 32 patients with pectus excavatum are shown in Table 43-3. Most significant are the cases of conduction blocks or arrhythmias. Patients with a history of palpitations should have a 24-hour electrocardiogram as well as an echocardiogram to evaluate for mitral valve prolapse. Resolution of these supraventricular arrhythmias has been anecdotally reported after correction of a pectus excavatum deformity.
Deformity of the chest wall led many authors to attribute the symptomatic improvement in patients after surgery to initial impairment in pulmonary function. This was difficult to prove, however, with the wide range of pulmonary function that exists from individual to individual and its dependence on physical training and body habitus.
Table 43-3 Electrocardiographic Findings in a Group of 32 Patients with Pectus Excavatum | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||
Pulmonary Function Studies
As early as 1951, Brown and Cook12 performed respiratory studies on patients before and after surgical repair. They demonstrated that although vital capacity (VC) was normal, the maximum breathing capacity was diminished (50% or more) in 9 of 11 cases and increased an average of 31% after surgical repair. Weg and associates102 evaluated 25 U.S. Air Force recruits with pectus excavatum and compared them with 50 unselected basic trainees. Although the lung compartments of both groups were equal, as were the VCs, the maximum voluntary ventilation was significantly lower in those with pectus excavatum than in the control population. Castile and coworkers15 evaluated seven patients with pectus excavatum, five of whom were symptomatic with exercise. The mean total lung capacity of the group was 79% of predicted. Flow volume configurations were normal, excluding airway obstruction as a cause of the symptoms. Workload tests demonstrated normal response to exercise in the ratio of dead space to tidal volume and alveolar–arterial oxygen difference. The measured oxygen uptake, however, increasingly exceeded predicted values as workload approached maximum in the four “symptomatic” subjects with pectus excavatum. This pattern of oxygen consumption was different from that in normal subjects and in the three asymptomatic subjects with pectus excavatum, in whom a linear response was seen. The mean oxygen uptake in the symptomatic subjects at maximal effort exceeded the predicted values by 25.4%. The three asymptomatic subjects, on the other hand, demonstrated normal linear oxygen uptake during exercise. Increased oxygen uptake suggests increased work of breathing in these symptomatic individuals despite the normal or mildly reduced VCs. Increases in tidal volume with exercise were uniformly depressed in those with pectus excavatum.
Cahill and coworkers13 performed pre- and postoperative studies in 5 children and adolescents with pectus carinatum and in 14 with pectus excavatum. No abnormalities were demonstrated in the pectus carinatum group. The low-normal VCs in excavatum patients were unchanged by operation, but a small improvement in the total lung capacity and a significant
improvement in the maximal voluntary ventilation were seen. Exercise tolerance improved in those with pectus excavatum after operation as determined both by total exercise time and maximal oxygen consumption. In addition, at any given workload, those with pectus excavatum demonstrated a lower heart rate, stable oxygen consumption, and higher minute ventilation after repair. Mead and associates60 studied rib cage mobility by assessing intra-abdominal pressure. Normal abdominal pressure tracings in pectus excavatum suggested normal rib cage mobility.
improvement in the maximal voluntary ventilation were seen. Exercise tolerance improved in those with pectus excavatum after operation as determined both by total exercise time and maximal oxygen consumption. In addition, at any given workload, those with pectus excavatum demonstrated a lower heart rate, stable oxygen consumption, and higher minute ventilation after repair. Mead and associates60 studied rib cage mobility by assessing intra-abdominal pressure. Normal abdominal pressure tracings in pectus excavatum suggested normal rib cage mobility.
Blickman and colleagues10 assessed pulmonary function in 17 children with pectus excavatum by xenon perfusion and ventilation scintigraphy before and after surgery. Ventilation studies were abnormal in 12 children before surgery and improved in 7 after repair. Perfusion scans were abnormal in 10 children before surgery and improved after operation in 6. The ventilation/perfusion ratios were abnormal in 10 of the 17 children preoperatively and normalized after repair in 6.
Derveaux and colleagues21 evaluated 88 patients with pectus excavatum and carinatum by pulmonary function tests (PFTs) before and 1 to 20 years after repair (mean 8 years). The surgical technique used a fairly extensive chest wall dissection. Preoperative studies were within the normal range (>80% of predicted) except in subjects with both scoliosis and pectus excavatum. The postoperative values for FEV1 and VC were decreased in all groups when expressed as a percentage of predicted, although the absolute values at follow-up may have been greater than at preoperative evaluation. Radiologic evaluation of these individuals confirmed improved chest wall configuration, so the relative deterioration in pulmonary function was not the result of recurrence of the pectus deformity. An inverse relationship was found between preoperative and postoperative function. Those with less than 75% of predicted function had improved function after surgery, but results were worse after repair if the preoperative values were greater than 75% of predicted. Almost identical results were found in a study by Morshuis and associates,64 who evaluated 152 subjects before and a mean of 8 years after surgery for pectus excavatum. These physiologic results were in contrast to the subjective improvement in symptoms from the subjects and the improved chest wall configuration. The decline in pulmonary function in the postoperative studies was attributed to the operation because the preoperative defect appeared to be stable regardless of the age at initial repair. Both studies were marred by the obvious lack of an age- and severity-matched control group without surgery.
Derveaux and colleagues20 evaluated transpulmonary and transdiaphragmatic pressures at total lung capacity in 17 individuals with pectus excavatum. Preoperative and long-term follow-up evaluations were performed a mean of 12 years apart. Reduced transpulmonary and transdiaphragmatic pressures suggested that the increased restrictive defect was produced by extrapulmonary rather than pulmonary factors, or that surgery produced increased rigidity of the chest wall.
Wynn and colleagues107 assessed 12 children with pectus excavatum by pulmonary function tests and exercise testing. Eight children had repair and were evaluated pre- and postoperatively. Four children had two sets of evaluations, but no operation. A decline in total lung capacity was identified in the repaired children compared with stable values in the control group. Cardiac output and stroke volume increased appropriately with exercise before and after operation in both groups, and the operation was believed to have produced no physiologically significant effect on the response to exercise.
Kaguraoka and associates47 evaluated pulmonary function in 138 individuals preceding and after repair of pectus excavatum. A decrease in VC occurred during the first 2 months after surgery, with recovery to preoperative levels by 1 year after operation. At 42 months, the values were maintained at baseline, despite a significant improvement in the chest wall configuration. Tanaka and coworkers94 found similar results in individuals who had the more extensive sternal turnover technique; in fact, they demonstrated a more significant and long-term decrease in VC. Morshuis and coworkers65 evaluated 35 subjects who had had pectus excavatum repaired as teenagers or young adults; ages were 17.9 ± 5.6 years. Preoperative evaluations were performed and repeated 1 year after surgery. Preoperative total lung capacity (86.0 ± 14.4% of predicted) and VC (79.7 ± 16.2%) were significantly decreased from predicted values and decreased further after surgery (9.2 ± 9.2% and 6.6 ± 10.7%, respectively). The efficiency of breathing at maximal exercise improved significantly after surgery. Ventilatory limitation of exercise occurred in 43% of the subjects before repair, and there was a tendency toward improvement after surgery. However, the group with no ventilatory limitation initially demonstrated a limitation after surgery with a significant increase in oxygen consumption.
Quigley and colleagues73 evaluated 36 adolescents with pectus excavatum and 10 age-matched healthy controls at baseline and then an average of 8 months after surgery in 15 subjects and 9 months in controls. Adolescents with pectus excavatum had a decrease in VC compared with controls, although the mean values remained in the normal range. The mean total lung capacity was normal. There was no difference in workload between subjects with pectus excavatum and the controls, with both groups achieving a similar duration and level of exercise. No significant change in follow-up pulmonary function tests was seen in either group. The duration of exercise as well as the level of work increased significantly in those who had surgery but not in the controls. The absence of adverse effects on pulmonary function after surgery was attributed to a less extensive surgical procedure than was used in the studies reported by Derveaux20 and Morshuis64,65 and their colleagues.
Results of pulmonary function tests in children having the minimally invasive repair of pectus excavatum (MIRPE) have provided similar results. Sigalet and coauthors92 assessed the pulmonary function in eleven patients preoperatively and at 3 months after surgery. FVC, VO2 max, and anaerobic threshold were all decreased significantly despite the patients reporting subjective improvement in their breathing. Similar results were reported in a prospective study by Aronson and coauthors3 in 145 patients having a MIRPE. There was no improvement in static values 6 months after removal of the struts.
In composite, these studies of pulmonary function over the past four decades have failed to document consistent improvement in pulmonary function resulting from surgical repair. In fact, studies have demonstrated deterioration in pulmonary function at long-term evaluation that was attributable to increased chest wall rigidity after surgical repair, and a meta-analysis by Malek and coworkers56a of 12 of these studies revealed no statistically significant change in pulmonary function. Despite this finding, workload studies have shown improvement
in exercise tolerance after repair, suggesting that there is a cardiac basis for this enhanced performance.
in exercise tolerance after repair, suggesting that there is a cardiac basis for this enhanced performance.
Cardiovascular Studies
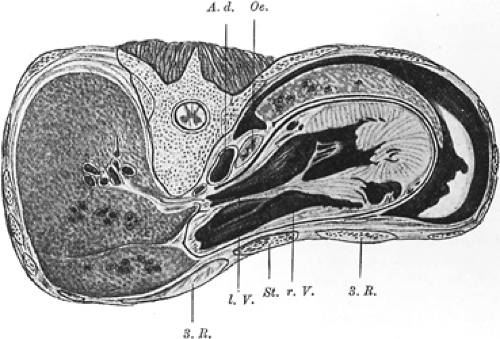
Posterior displacement of the sternum can produce a deformity of the heart, particularly anterior indentation of the right ventricle. Early pathologic studies demonstrated this finding (Fig. 43-3). By angiography, Garusi and D’Ettorre28 showed displacement of the heart to the left, often with a sternal imprint on the anterior wall of the right ventricle. Also by angiography, Howard41 demonstrated its resolution by surgical repair. Elevated right heart pressures have been reported by some researchers, as have pressure curves similar to those seen in constrictive pericarditis. In 1962, Bevegård8 studied 16 individuals with pectus excavatum by right heart catheterization and exercise testing. The physical work capacity in pectus excavatum at a given heart rate was significantly lower in the sitting than the supine position. Those with 20% or greater decline in physical work capacity from the supine to the sitting position had shorter sternovertebral distances than did those with less decrease in their physical work capacity. The measured stroke volume at rest decreased from supine to sitting positions a mean of 40.3%, similar to normal subjects. In the supine position, stroke volume increased with exercise to 13.2%. In the sitting position, the increase in stroke volume from rest to exercise was 18.5% for the pectus excavatum group, significantly lower (p < 0.001) than the 51% increase seen in normal subjects. Thus, in the pectus excavatum group, an increased cardiac output could be achieved primarily by increased heart rate because limited enhancement of the stroke volume could occur. Intracardiac pressures measured at rest and with exercise were normal in all subjects despite this apparent limitation of ventricular volume. Gattiker and Bühlmann29 confirmed this limitation of the stroke volume in a study of 19 subjects. In the upright position at a heart rate of 170 beats per minute, the physical work capacity was lower than in the supine position (mean 18% decrease) because of the decrease in stroke volume. Beiser and associates7 performed cardiac catheterization in six adolescents and young adults with moderate degrees of pectus excavatum. Normal pressure and cardiac index were obtained at rest in the supine position. The cardiac index during moderate exercise was normal, but the response to upright exercise was below that predicted in two patients and at the lower limit of normal in three. The cardiac index was 6.8 ± 0.8 L/min per square meter compared with 8.9 ± 0.3 L/min per square meter in a group of 16 normal controls (p <0.01). The difference in cardiac performance again appeared to be produced primarily by a smaller stroke volume in the group in an upright position. Stroke volume was 31% lower and cardiac output 28% lower during upright as compared with supine exercise. Postoperative studies were performed in three individuals: two of these achieved a higher level of exercise tolerance after surgery. The cardiac index increased an average of 38%. Because heart rate at maximal exercise was not higher after repair, an enhanced stroke-volume response was responsible for this increase.
Peterson and associates72 performed radionuclide angiography and exercise studies in 13 children with pectus excavatum. Of these, 10 were able to reach the target heart rate before surgical repair, 4 without symptoms. After operation, all but one child reached the target heart rate during the exercise protocol, and 9 of 13 reached the target without becoming symptomatic. The left and right ventricular end-diastolic volumes were consistently increased after repair at rest, and the mean stroke volume was increased 19% after repair. These findings substantiated the changes in ventricular volume previously demonstrated by cardiac catheterization, although an increase in the cardiac index was not demonstrated. Recent echocardiographic studies by Kowalewski and associates49 of 42 patients before and 6 months after surgery revealed statistically significant changes in the right ventricular volume indices after surgery. However, no correlation was seen between the pectus index and the changes in the right ventricular volume indices.
Malek and coauthors56 performed maximal exercise testing and PFTs on 21 individuals with pectus excavatum, 18 of whom routinely did aerobic exercise. Their maximum oxygen uptake and oxygen pulse were both significantly lower than expected and were attributed to cardiovascular and not ventilatory limitation and resulted in an abnormally low threshold for lactate accumulation. Of note, subjects with a Haller index >4.0 were eight times more likely to have reduced aerobic capacity than those with a lower severity index.
Results in terms of cardiac function have more recently been assessed in patients having the MIRPE procedure. Sigalet and coauthors92 showed, in 11 patients, an increase in the stroke volume measured echocardiographically 3 months after repair; subjective improvement in exercise tolerance was attributed to this.
Malek and colleagues57 performed a meta-analysis of eight studies (representing 169 patients), which provided quantitative data on the cardiovascular function of patients before and following repair. They concluded that surgical repair did significantly improve cardiovascular function.
Additional studies are needed to further define the relationship between pectus excavatum and cardiopulmonary function. Recent dynamic or exercise studies have been most promising in this area. Methods to more effectively evaluate preoperative cardiopulmonary function are needed to identify which children may achieve symptomatic and physiologic improvement from surgical repair.
Echocardiographic Studies
Bon Tempo,11 Salomon,79 and Schutte85 and their associates reported mitral valve prolapse in patients with narrow anterior-posterior chest diameters, anterior chest wall deformities, and scoliosis. Echocardiographic prospective studies of adults with pectus excavatum demonstrated mitral valve prolapse in 6 of 33 (18%) subjects studied by Udoshi and associates95 and in 11 of 17 subjects (65%) of Saint-Mezard and colleagues.78 Anterior compression of the heart by the depressed sternum may deform the mitral annulus or the ventricular chamber and produce mitral valve prolapse in these patients. Preoperative evaluation by echocardiogram of children with pectus excavatum by Shamberger and associates88 identified 23 children with mitral valve prolapse. Postoperative studies did not demonstrate mitral valve prolapse in 10 (43%) of these children, suggesting resolution after correction of the chest wall deformity.
Coln and coauthors16 performed echocardiography on subjects during exercise (123 preop and 107 postop). They demonstrated chamber compression in 117 (95%) before surgery and in none of the patients after repair. They also found in subjects with chamber compression resolution of mitral valve prolapse (16 of 23) and mitral valve regurgitation (28 of 29) following repair. Symptoms related to exertion reported in 106 (86%) of the subjects resolved remarkably in all, which was attributed to the cardiac effects of repair.
Quality-of-Life Analysis
Questionaires have been developed to look at the impact of surgical repair of pectus excavatum on the quality of life related to both physical and psychosocial functioning. Lawson and associates51 used these tools to interview both children and parents. Children indicated significant improvement in frequency of experiencing exercise tolerance, shortness of breath, and fatigue. Parents reported similar improvements. All indicators of psychosocial functioning improved.
Surgical Repair
The first surgical corrections of pectus excavatum were reported by Meyer in 191161 and Sauerbruch in 1920. In 1939, Ochsner and DeBakey summarized their early experiences with various techniques.67 In 1949, Ravitch74 reported a technique that included excision of all deformed costal cartilages with the perichondrium, division of the xiphoid from the sternum, division of the intercostal bundles from the sternum, and a transverse sternal osteotomy securing the sternum anteriorly in an overcorrected position. He used Kirschner wire fixation in the first two patients and silk suture fixation in later patients.
Baronofsky5 and Welch103 reported a technique for the correction of pectus excavatum that emphasized total preservation of the perichondrial sheaths as well as the attachment of the upper sheaths and intercostal bundles to the sternum. Anterior fixation of the sternum was achieved with silk sutures. The technique I use today remains unchanged from these methods except for the use of retrosternal strut fixation in all children. Haller and associates32 later developed a technique called tripod fixation, in which subperichondrial resection of the abnormal cartilages is performed followed by a posterior sternal osteotomy. The most cephalad normal cartilages are then divided obliquely in a posterolateral direction. When the sternum is elevated, the sternal ends of the cartilage rest on the costal ends, providing further anterior support of the sternum.
Several researchers have promoted supporting the sternum by metallic struts after mobilization of the costal cartilages. Rehbein and Wernicke76 developed struts that could be placed into the marrow cavity of the ribs at the costochondral junction. An arch was then formed by the struts anterior to the sternum, and the sternum was secured to this arch. Paltia and associates71 placed a transverse strut through the caudal end of the sternum, firmly fixing its location. The two ends of the strut are supported by the ribs laterally. Adkins and Blades1 and Jensen and associates44 used retrosternal elevation by a metallic strut. Willital106 used a similar retrosternal strut after creating multiple chondrotomies in the costal cartilages to provide flexibility. Recent innovations in these methods include bioabsorbable struts, Marlex mesh, or a Dacron vascular graft as a strut, but there is no evidence that these methods are preferable to traditional methods. No randomized studies have compared the recurrence or complication rates between suture or strut fixation techniques. Oelsnitz68 and Hecker and coworkers,38 using suture fixation, reported satisfactory repairs in their large series in 90% to 95% of patients.
The sternal turnover was first proposed by Judet and Judet45 and Jung46 in the French literature. In this method, the sternum is mobilized and the costal cartilages are divided, allowing the sternum to be rotated 180 degrees. Wada and colleagues98 have reported a large series from Japan using this technique, which is essentially a free graft of sternum. It is a radical approach and has been associated with major complications if infection occurs. Modifications of this technique by Taguchi and associates93 have involved either preservation of the internal mammary vessels by wide dissection or reimplantation of the internal mammary artery. These methods were developed because of the reported incidence of osteonecrosis and fistula formation, which occurred in up to 46% of patients over 15 years of age.
A method described by Allen and Douglas2 is that of implantation of Silastic molds into the subcutaneous space to fill the deformity. Margulis and colleagues58 have recently summarized their favorable results with currently available implants. Although this approach may improve the external contour of the chest, extrusion of the molds has occurred, and this method does nothing to increase the volume of the thoracic cavity or relieve compression on the heart.
Schier84 described use of a suction device placed over the chest in children and adults with pectus excavatum and early results were encouraging, although the durability of the correction was not established. Haecker and Mayr31 described a similar device. Although improvement was seen, only 14.7% of the patients had the sternum lifted to a “normal” position after 12 months of therapy.
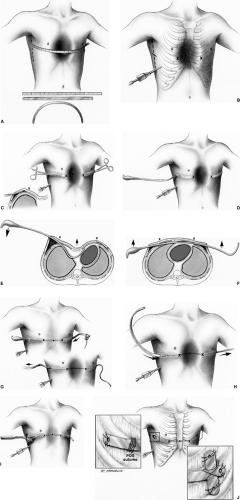
A method of elevation of the sternum with a retrosternal bar without resection or division of the costal cartilages was reported by Nuss and associates66 and has subsequently been labeled either the minimally invasive repair of pectus excavatum (MIRPE) or the Nuss procedure (Fig. 43-4). He repaired 42 patients under 15 years of age (median age 5 years) by placing a convex steel bar under the sternum and anterior to the heart through small bilateral thoracic incisions. As initially described, a long clamp was passed blindly behind the sternum and out the contralateral opening. A tape was then drawn across and used to
pull the bar through the chest. The bar is initially placed with the concave side anteriorly and then it is rotated once in position (Fig. 43-4). The bar is left in position for 3 years before removal, when presumed permanent remodeling of the cartilages has occurred. Although Nuss, in his initial report, warned that the “upper limits of age for this procedure require further evaluation,” the technique has been widely used in older patients and long-term results from this population have not yet been reported. Hebra and colleagues37 have reported the use of this technique in adults. In 2002, the results by Nuss and his associates18 using this technique in 303 patients were reported. This included an older group of children than in the initial report (range 21 months to 29 years; median age 12.4 years). Two bars were required in 12.5% of the patients. Routine use of thoracoscopy to avoid cardiac injury was instituted in 1998. Lateral stabilizers were placed in 69.4% of the cases; they were routinely used after 1998 and were wired to the bar in 65.4% of cases (Fig. 43-4D). Epidural analgesic was used for 2 to 4 days and the median length of stay was 5 days, with a range of 3 to 10 days. The frequency of early complications was low. It included pneumothorax requiring aspiration, 1.0%; pericarditis, 2.3% (with only 0.3% requiring drainage); pneumonia, 0.7%; hemothorax, 0.3%; transient extremity paralysis, 0.3%; superficial wound infection, 2.3%; and bar infection requiring eventual removal of the bar, 0.7%.
pull the bar through the chest. The bar is initially placed with the concave side anteriorly and then it is rotated once in position (Fig. 43-4). The bar is left in position for 3 years before removal, when presumed permanent remodeling of the cartilages has occurred. Although Nuss, in his initial report, warned that the “upper limits of age for this procedure require further evaluation,” the technique has been widely used in older patients and long-term results from this population have not yet been reported. Hebra and colleagues37 have reported the use of this technique in adults. In 2002, the results by Nuss and his associates18 using this technique in 303 patients were reported. This included an older group of children than in the initial report (range 21 months to 29 years; median age 12.4 years). Two bars were required in 12.5% of the patients. Routine use of thoracoscopy to avoid cardiac injury was instituted in 1998. Lateral stabilizers were placed in 69.4% of the cases; they were routinely used after 1998 and were wired to the bar in 65.4% of cases (Fig. 43-4D). Epidural analgesic was used for 2 to 4 days and the median length of stay was 5 days, with a range of 3 to 10 days. The frequency of early complications was low. It included pneumothorax requiring aspiration, 1.0%; pericarditis, 2.3% (with only 0.3% requiring drainage); pneumonia, 0.7%; hemothorax, 0.3%; transient extremity paralysis, 0.3%; superficial wound infection, 2.3%; and bar infection requiring eventual removal of the bar, 0.7%.
Late complications in this series included bar displacement requiring repositioning in 8.6%, which included a high proportion (over 50%) of patients in whom a stabilizer was not used or
patients where the stabilizer was not wired to the bar. When both modifications were used, displacement occurred in only 5% of the patients. Late hemothorax occurred in two patients, one secondary to trauma, in which the source was not defined. The occurrence of a mild overcorrection in the deformity was seen in 3.6% and a pectus carinatum deformity developed in 1.3%, all of whom had either Marfan’s syndrome or Ehlers–Danlos syndrome. The outcome of children in this large series was good, with excellent appearance in 84.5%, good in 14.8%, and failed in only one patient, but the bars had been removed at the time of the report in only 23.4% of the patients. A more recent summary by Shin and associates90 of the infectious complications in a consecutive series of 863 patients showed a 1.5% incidence. This included six bar infections, four cases of cellulitis, and three stitch abscesses. Antibiotics and surgical drainage resolved the infection in three of the bar infections, while removal of the bar was required in three, one 3 months after surgery, and two 18 months after surgery. Organisms involved were predominantly Staphylococcus aureus in 83% of the cases. This success of preserving the struts despite infectious complications was mirrored by the experience of Van Renterghem and associates97 and Calkins and coauthors14 as well.
patients where the stabilizer was not wired to the bar. When both modifications were used, displacement occurred in only 5% of the patients. Late hemothorax occurred in two patients, one secondary to trauma, in which the source was not defined. The occurrence of a mild overcorrection in the deformity was seen in 3.6% and a pectus carinatum deformity developed in 1.3%, all of whom had either Marfan’s syndrome or Ehlers–Danlos syndrome. The outcome of children in this large series was good, with excellent appearance in 84.5%, good in 14.8%, and failed in only one patient, but the bars had been removed at the time of the report in only 23.4% of the patients. A more recent summary by Shin and associates90 of the infectious complications in a consecutive series of 863 patients showed a 1.5% incidence. This included six bar infections, four cases of cellulitis, and three stitch abscesses. Antibiotics and surgical drainage resolved the infection in three of the bar infections, while removal of the bar was required in three, one 3 months after surgery, and two 18 months after surgery. Organisms involved were predominantly Staphylococcus aureus in 83% of the cases. This success of preserving the struts despite infectious complications was mirrored by the experience of Van Renterghem and associates97 and Calkins and coauthors14 as well.
Metal allergy to the retrosternal bars was also identified in 2.2% of the patients having MIRPE by Nuss and his group.77 The majority (63%) presented with rash and erythema while 32% had pleural effusions and 15% were diagnosed on preoperative screening. It is obviously critical to distinguish between allergic and infectious complications.
The importance of placing the bars and stabilizers in a subcutaneous and not a submuscular position to avoid extra osseous bone formation around the strut and increased blood loss at the time of removal has been demonstrated.89
Hebra and associates35 reported the results of a survey of members of the American Pediatric Surgery Association who had used the minimally invasive (Nuss) technique. Thirty institutions contributed their cases, which totaled 251, although it should be noted that 42% were performed by one surgeon. The complications reported were similar to those of Nuss and his associates, but the frequency was higher, presumably because the procedures were performed by more individuals less familiar with the operation. Displacement of the bar occurred in 9.2% of cases and pneumothorax requiring tube thoracostomy in 4.8%. Less frequently encountered complications included thoracic outlet syndrome, pericarditis, blood loss requiring a transfusion, cardiac injury, persistent cardiac arrhythmias, and erosion of the sternum by the bar. Many of the surgeons had adopted the use of thoracoscopy to improve the safety of passing the clamp anterior to the heart. Other surgeons elevate the sternum with a bone hook during passage of the clamp to open the retrosternal space anterior to the heart.
Engum and coworkers23 reported their series of 21 patients with a mean age of 8.2 years. Their patients had an average hospital stay of 4.9 days, which was comparable with that for the open repair. Complications encountered in their series were similar to the experience of others and included rotation of the bar, production of a marked pectus carinatum deformity,
progressive chest wall asymmetry, and chronic, persistent pain requiring removal of the bar in one case.
progressive chest wall asymmetry, and chronic, persistent pain requiring removal of the bar in one case.
Molik and colleagues63 later enlarged this single-institution review and in a retrospective review compared 68 patients who underwent a standard surgical repair with 35 patients who underwent a Nuss repair. The Nuss procedure required less time (3.3 hours) compared with the open technique (4.7 hours) but had a higher complication rate (43%) than the open method (20%). In all, four patients with the standard operation (6%) and eight with the Nuss technique (29%) required reoperation. Length of stay was comparable between the open (4.8 days) and Nuss (4.0 days) techniques. The Nuss patients had a higher frequency of epidural analgesics postoperatively and an increased duration of patient-controlled analgesia after surgery.
Fonkalsrud and associates24 reported a similar retrospective comparison of the two techniques, each utilized at a single institution: 68 patients had the minimally invasive procedure and 139 had the open technique during a 5-year period. There was a higher incidence of reoperations and hospitalizations in the Nuss group, but it was noted that 90% of the complications of this technique occurred in the first 25 cases, again clearly demonstrating the role of experience in determining the frequency of surgical complications. It was difficult to differentiate whether the differences noted in the use of epidural catheters and intravenous narcotics were attributable to distinct patient requirements or institutional bias. There was a shorter mean hospitalization noted for the open procedure (2.9 days) compared with the minimal access procedure (6.5 days) and a similar difference between mean time before return to work or school (12 versus 18 days). These investigators concluded that “long-term follow-up also will be required to assure both health professionals and the public that this is the procedure of choice for patients with pectus excavatum.”
The occurrence of “overcorrection” of the deformity or production of a true carinate deformity was first reported by Croitoru and associates18 and was associated with underlying connective tissue disorders (Marfan’s and Ehlers–Danlos syndromes). It was reported, however, by Hebra,36 to occur in a healthy 13-year-old boy 1 year after Nuss repair. What factors predispose some patients to this complication is not understood.
Other rare complications for the MIRPE include those reported by Leonhardt and associates53 of exsanguinating hemorrhage during removal of the strut resulting from laceration of a pulmonary vessel and bilateral sternoclavicular dislocation. Hoel and colleagues39 reported cardiac tamponade and shock produced by erosion of the aorta by a strut that had rotated 90 degrees, and Barsness and associates6 reported a near-fatal hemorrhage from erosion of the internal mammary vessel by a bar that had rotated 45 degrees.
A prospective multi-institutional study of patients undergoing repair of pectus excavatum has completed enrollment. It is hoped that this study will better define the role as well as the risks and benefits of the open and minimally invasive surgical procedures in the repair of pectus excavatum and the long-term effects on cardiopulmonary function. Early reports by Kelly and coauthors48 from this study suggest that the pain and complications from the MIRPE and open procedures are similar and can be done with limited risks to the patients.
Children should be followed long-term after repair by any technique until they reach full stature. Only by so doing can each surgeon assess the ultimate results of his or her surgical technique. Regrettably, there can be recurrence until full stature is achieved.
Surgical Technique
The open surgical technique for correction of pectus excavatum is depicted in Figure 43-5. In girls, particular attention is focused on placing the incision within the projected inframammary crease, thus preventing the complications of breast deformity and development described by Hougaard and Arendrup.40 Skin flaps are mobilized by electrocautery to the angle of Louis superiorly and to the xiphoid inferiorly. Pectoral muscle flaps are elevated off the sternum and costal cartilages, thus preserving the entire pectoralis major and portions of the pectoralis minor and serratus anterior muscles in the flap (Fig. 43-5A). Ellis and associates22 have described elevating the skin and muscle together in a single flap, which is a reasonable but not widely adopted alternative method.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree