CHAGAS’ DISEASE
Although the genus Trypanosoma contains many species of protozoans, only T. cruzi, T. brucei gambiense, and T. brucei rhodesiense cause disease in humans. T. cruzi is the etiologic agent of Chagas’ disease in the Americas; T. b. gambiense and T. b. rhodesiense cause African trypanosomiasis.
CHAGAS’ DISEASE
DEFINITION
Chagas’ disease, or American trypanosomiasis, is a zoonosis caused by the protozoan parasite T. cruzi. Acute Chagas’ disease is usually a mild febrile illness that results from initial infection with the organism. After spontaneous resolution of the acute illness, most infected persons remain for life in the indeterminate phase of chronic Chagas’ disease, which is characterized by subpatent parasitemia, easily detectable antibodies to T. cruzi, and an absence of associated signs and symptoms. In 10–30% of chronically infected patients, cardiac and/or gastrointestinal lesions develop that can result in serious morbidity and even death.
LIFE CYCLE AND TRANSMISSION
T. cruzi is transmitted among its mammalian hosts by hematophagous triatomine insects, often called reduviid bugs. The insects become infected by sucking blood from animals or humans who have circulating parasites. Ingested organisms multiply in the gut of the triatomines, and infective forms are discharged with the feces at the time of subsequent blood meals. Transmission to a second vertebrate host occurs when breaks in the skin, mucous membranes, or conjunctivae become contaminated with bug feces that contain infective parasites. T. cruzi can also be transmitted by the transfusion of blood donated by infected persons, by organ transplantation, from mother to unborn child, by ingestion of contaminated food or drink, and in laboratory accidents.
PATHOLOGY
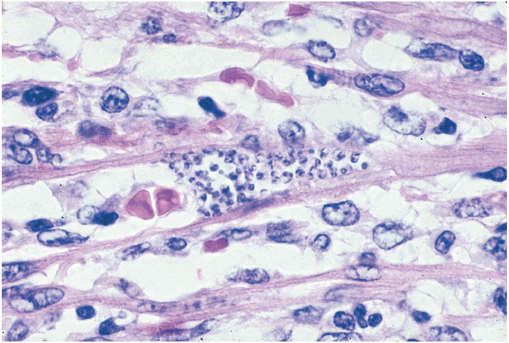
Initial infection at the site of parasite entry is characterized by local histologic changes that include the presence of parasites within leukocytes and cells of subcutaneous tissues and the development of interstitial edema, lymphocytic infiltration, and reactive hyperplasia of adjacent lymph nodes. After dissemination of the organisms through the lymphatics and the bloodstream, primarily muscles (including the myocardium) (Fig. 27-1) and ganglion cells may become heavily parasitized. The characteristic pseudocysts present in sections of infected tissues are intracellular aggregates of multiplying parasites.
FIGURE 27-1
Trypanosoma cruzi in the heart muscle of a child who died of acute Chagas’ myocarditis. An infected myocyte containing several dozen T. cruzi amastigotes is in the center of the field (hematoxylin and eosin, 900×).
In individuals with chronic T. cruzi infections who develop related clinical manifestations, the heart is the organ most commonly affected. Changes include thinning of the ventricular walls, biventricular enlargement, apical aneurysms, and mural thrombi. Widespread lymphocytic infiltration, diffuse interstitial fibrosis, and atrophy of myocardial cells are often apparent, but parasites are difficult to find in myocardial tissue by conventional histologic methods. Conduction-system abnormalities often affect the right branch and the left anterior branch of the bundle of His. In chronic Chagas’ disease of the gastrointestinal tract (megadisease), the esophagus and colon may exhibit varying degrees of dilatation. On microscopic examination, focal inflammatory lesions with lymphocytic infiltration are seen, and the number of neurons in the myenteric plexus may be markedly reduced. Accumulating evidence implicates the persistence of parasites and the accompanying chronic inflammation—rather than autoimmune mechanisms—as the basis for the pathology in patients with chronic T. cruzi infection.
EPIDEMIOLOGY
T. cruzi is found only in the Americas. Wild and domestic mammals harboring T. cruzi and infected triatomines are found in spotty distributions from the southern United States to southern Argentina. Humans become involved in the cycle of transmission when infected vectors take up residence in the primitive wood, adobe, and stone houses common in much of Latin America. Thus, human T. cruzi infection is a health problem primarily among the poor in rural areas of Mexico and Central and South America. Most new T. cruzi infections in rural settings occur in children, but the incidence is unknown because most cases go undiagnosed. Historically, transfusion-associated transmission of T. cruzi was a serious public health problem in many endemic countries. However, with some notable exceptions, transmission by this route has been essentially eliminated as effective programs for the screening of donated blood have been implemented. Several dozen patients with HIV and chronic T. cruzi infections who underwent acute recrudescence of the latter have been described. These patients generally presented with T. cruzi brain abscesses, a manifestation of the illness that does not occur in immunocompetent persons. Currently, it is estimated that 8 million people are chronically infected with T. cruzi and that 14,000 deaths due to the illness occur each year. The resulting morbidity and mortality make Chagas’ disease the most important parasitic disease burden in Latin America.
In recent years, the rate of T. cruzi transmission has decreased markedly in several endemic countries as a result of successful programs involving vector control, blood-bank screening, and education of at-risk populations. A major program, which began in 1991 in the “southern cone” nations of South America (Uruguay, Paraguay, Bolivia, Brazil, Chile, and Argentina), has provided the framework for much of this progress. Uruguay and Chile were certified free of transmission by the main domiciliary vector species (Triatoma infestans) in the late 1990s, and Brazil was declared transmission-free in 2006. Transmission has been reduced markedly in Argentina as well. Similar control programs have been initiated in the countries of northern South America and in the Central American nations.
Acute Chagas’ disease is rare in the United States. Five cases of autochthonous transmission and five instances of transmission by blood transfusion have been reported. Moreover, T. cruzi was transmitted to five recipients of organs from three T. cruzi–infected donors. Two of these recipients became infected through cardiac transplants. Acute Chagas’ disease has not been reported in tourists returning to the United States from Latin America, although three such instances have been reported in Europe. In contrast, the prevalence of chronic T. cruzi infections in the United States has increased considerably in recent years. An estimated 23 million immigrants from Chagas’-endemic countries currently live in the United States, ~17 million of whom are Mexicans. The total number of T. cruzi–infected persons living in the United States is estimated to be 300,000. Screening of the U.S. blood supply for T. cruzi infection began in January 2007. The overall prevalence of T. cruzi infection among donors is about 1 in 29,000, and to date more than 1200 infected donors have been identified and deferred permanently (see “Diagnosis,” later in this chapter).
CLINICAL COURSE
The first signs of acute Chagas’ disease develop at least 1 week after invasion by the parasites. When the organisms enter through a break in the skin, an indurated area of erythema and swelling (the chagoma), accompanied by local lymphadenopathy, may appear. Romaña’s sign—the classic finding in acute Chagas’ disease, which consists of unilateral painless edema of the palpebrae and periocular tissues—can result when the conjunctiva is the portal of entry. These initial local signs may be followed by malaise, fever, anorexia, and edema of the face and lower extremities. Generalized lymphadenopathy and hepatosplenomegaly may develop. Severe myocarditis develops rarely; most deaths in acute Chagas’ disease are due to heart failure. Neurologic signs are not common, but meningoencephalitis occurs occasionally, especially in children <2 years old. Usually within 4–8 weeks, acute signs and symptoms resolve spontaneously in virtually all patients, who then enter the asymptomatic or indeterminate phase of chronic T. cruzi infection.
Symptomatic chronic Chagas’ disease becomes apparent years or even decades after the initial infection. The heart is commonly involved, and symptoms are caused by rhythm disturbances, segmental or dilated cardiomyopathy, and thromboembolism. Right bundle-branch block is a common electrocardiographic abnormality, but other types of intraventricular and atrioventricular blocks, premature ventricular contractions, and tachy- and bradyarrhythmias occur frequently. Cardiomyopathy often results in biventricular heart failure with a predominance of right-sided failure at advanced stages. Embolization of mural thrombi to the brain or other areas may take place. Sudden death is the main cause of death in Chagas’ heart disease. Patients with megaesophagus suffer from dysphagia, odynophagia, chest pain, and regurgitation. Aspiration can occur (especially during sleep) in patients with severe esophageal dysfunction, and repeated episodes of aspiration pneumonitis are common. Weight loss, cachexia, and pulmonary infection can result in death. Patients with megacolon are plagued by abdominal pain and chronic constipation, which predisposes to fecaloma formation. Advanced megacolon can cause obstruction, volvulus, septicemia, and death.
DIAGNOSIS
The diagnosis of acute Chagas’ disease requires the detection of parasites. Microscopic examination of fresh anticoagulated blood or the buffy coat is the simplest way to see the motile organisms. Parasites also can be seen in Giemsa-stained thin and thick blood smears. Microhematocrit tubes containing acridine orange as a stain can be used for the same purpose. When used by experienced personnel, all of these methods yield positive results in a high proportion of cases of acute Chagas’ disease. Serologic testing plays no role in diagnosing acute Chagas’ disease.
Chronic Chagas’ disease is diagnosed by the detection of specific IgG antibodies that bind to T. cruzi antigens. Demonstration of the parasite is not of primary importance. In Latin America, ~30 assays are commercially available, including several based on recombinant antigens. Although these tests usually show good sensitivity and reasonable specificity, false-positive reactions may occur—typically with samples from patients who have other infectious and parasitic diseases or autoimmune disorders. In addition, confirmatory testing has presented a persistent challenge. For these reasons, the World Health Organization recommends that specimens be tested in at least two assays and that well-characterized positive and negative comparison samples be included in each run. The radioimmune precipitation assay (Chagas RIPA) is a highly sensitive and specific confirmatory method for detecting antibodies to T. cruzi (approved under the Clinical Laboratory Improvement Amendment and available in the authors’ laboratory). In December 2006, the U.S. Food and Drug Administration (FDA) approved a test to screen blood and organ donors for T. cruzi infection (Ortho T. cruzi ELISA Test System, Ortho-Clinical Diagnostics, Raritan, NJ). Since January 2007, the vast majority of U.S. blood donors have been screened with the Ortho test, and positive units have undergone confirmatory testing in the Chagas RIPA. A second test for donor screening was approved by the FDA in April 2010 (Abbott PRISM® Chagas Assay, Abbott Laboratories, Abbott Park, IL). The use of PCR assays to detect T. cruzi DNA in chronically infected persons has been studied extensively. The sensitivity of this approach has not been shown to be reliably greater than that of serology, and no PCR assays are commercially available.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree