Chagas’ Disease of the Esophagus
Manoel Ximenes III
Etiology
American trypanosomiasis is an endemic disease caused by the protozoan Trypanosoma cruzi, discovered by the Brazilian scientist Carlos Chagas.10 Soon after his discovery, having observed the frequency of the infection and dysphagia in rural areas of Brazil where the disease is endemic, Chagas postulated a link between esophageal symptoms and the parasite. Etzel,17 Correa Neto,11 and Ramos42 studied the association between the digestive tract “mega-” syndromes and involvement of the heart and other organs. The organism is transmitted to humans and other mammals through insects of the species Triatoma when they bite skin to suck blood. Transmission may also occur by transfusion of contaminated blood, congenitally, and during organ transplantation.
Trypanosomiasis has a distribution throughout the Americas, from latitude 42 degrees north (northern California) to latitude 45 degrees south (southern Argentina and Chile), but the human infection extends from the southern United States to the state of Chubut in Argentina. Only three authenticated cases have been described in the United States, but the large number of Latin American immigrants who may be contaminated with T. cruzi may become a source of contamination through blood transfusion or organ donation.
Ninety million people from a total population of 360 million in Latin America (25%) are at risk of contracting the disease. Only one-third of those infected may show signs of the disease (i.e., 4.8 to 5.4 million people), and of these, 10% to 12% have overt esophageal achalasia.
Triatomines of the order Hemiptera, family Reduviidae, and subfamily Triatominae that transmit this malady consist of more than 100 species; another 100 vertebrate hosts have been naturally infected with the parasite. Triatomines usually live in rural areas in easy contact with vertebrates (e.g., birds, reptiles, bats, and dogs); in recent years, however, new epidemiologic conditions include a peridomestic environment and the interiors of houses. Of all potential vectors of T. cruzi, five are of special importance, notably in South America: Triatoma infestans, T. brasiliensis, T. dimidiata, T. sordida, and Panstrongylus megistus. Rhodnius prolixus and R. pallescens are active in Central America and Panama.
In the United States, 10 species of Triatominae have been described, including T. sanguisuga in Illinois, T. protacta in the Midwest, and T. rubida uhleri and T. gerstaeckeri in Texas, Arizona, and New Mexico. However, these insects are not important in transmission in North America because they do not defecate right after a blood meal and they live away from the domestic environment. Figure 150-1 demonstrates the distribution of Triatominae on the continent.
Pathophysiology
Once the parasite penetrates the skin, a local skin reaction occurs and leads to a “septicemic” stage. The chronic phase follows, with manifestations of the disease in several target organs, including the heart, digestive tract, and nervous system.
Since the early studies by Hurst and Rake,27 it has been demonstrated that the most important changes in Chagas’ disease are related to the autonomic nervous system, especially the Auerbach plexus. Koberle and Nabor29 and Koberle and Alcantara,28 after painstaking studies, demonstrated that the loss of ganglion cells during the acute phase involves a neurotoxin. This theory has not been widely accepted, however; instead, mechanisms linked to cell immunity are now strongly favored. T. cruzi organisms may be directly responsible for the reduction and subsequent destruction of the autonomic nervous system and consequently for the pathologic alterations in the affected organs. Another described mechanism is related to the antigenicity of the parasite triggering an autoimmune response. Another possibility related to the immune mechanism is that the parasitic antigens bind to host cells, and these cells become targets of the host immune system. An allergic reaction might also be involved in the pathogenesis of the tissue lesions, based on the findings that no relation exists between the localization of the tissue lesions and the concentration of parasites and that the mononuclear infiltrate does not necessarily correspond to sites where the parasites are present.
According to Andrade and Andrade,2 infection by T. cruzi may assume different patterns in the several geographic areas in which the disease is seen. This variety results from different strains of parasites that may grow quickly (type I), at an intermediate rate (type II), and slowly (type III). Different strains of T. cruzi have been isolated by means of isoenzymes, schizodemes, and DNA probes. By gel electrophoresis, they were classified into groups with identical isoenzyme profiles. The World Health Organization55 has identified three types: zymodemes 1 and 3 were identified in reservoirs and vectors of the nondomestic cycle, and zymodeme 2 was isolated in infected patients with chronic Chagas’ disease. The existence of different strains may
explain why the same disease is associated with clinical findings that differ according to geography. For instance, in the central plateau of Brazil, the “mega-” syndromes are prevalent, whereas in Venezuela, the disease shows a preference for the heart; in the United States, Chagas’ disease is almost nonexistent.
explain why the same disease is associated with clinical findings that differ according to geography. For instance, in the central plateau of Brazil, the “mega-” syndromes are prevalent, whereas in Venezuela, the disease shows a preference for the heart; in the United States, Chagas’ disease is almost nonexistent.
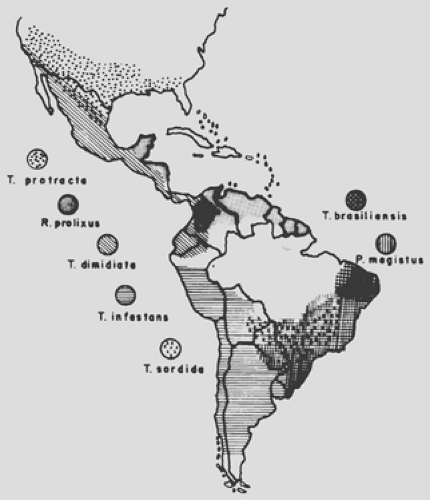 Figure 150-1. Geographic distribution of the main Triatominae vectors of Trypanosoma cruzei. (Courtesy of Z. Brener and Z.A. Andrade.) |
According to Koberle and Alcantara,28 the number of ganglions in the myenteric plexus in the normal esophagus varies from 220,000 to 250,000. Striated muscles are prevalent in the upper part of the esophagus, whereas smooth muscle predominates in the lower third. Ganglion cells are absent in the upper part and are found in numbers in the distal esophagus, thus explaining why dilatation occurs from the midesophagus down when ganglion cells are destroyed. When the proportion of parasympathetic ganglion cells lost reaches 90%, symptoms related to absence of peristalsis, loss of coordination, and achalasia with distention, hypertrophy, and hyperplasia of the muscle fibers appear. The thresholds for the development of symptoms of the other organs are 20% destruction of ganglion cells in the heart, 60% in the sigmoid esophagus, and near 80% in the bronchi.
No difference is noted in the number of lost ganglion cells in either the idiopathic achalasia found in Europe and North America or the megaesophagus related to Chagas’ disease. Differences between Chagas’ megaesophagus and idiopathic achalasia are related to the fact that the former always appears in the endemic areas of Latin America and is associated with other affected organs (heart, digestive system) and that serologic tests are positive for Chagas’ disease more than 90% of the time. The end results being the same, identical surgical treatment can be applied to both conditions.
Clinical Features
When it affects the esophagus, Chagas’ disease is usually seen 7 to 10 years after the primary infection. According to Rezende,46 the disease is seen in patients between 20 and 40 years of age, with a prevalence in men (65%) from an endemic area. With the current trend of migration into the cities, many of these patients are seen in major urban centers.
Once an individual has become infected, three stages of the disease may be elicited: acute, intermediate asymptomatic, and symptomatic. The incubation period usually lasts 1 week, during which two signs may be seen: chagoma (hardening of the skin with plates of red color) at the site where the bug has bitten and Romaña’s sign if the infection takes place in the orbital area. Only 1% to 2% of infected persons show signs of the infection; the remainder are asymptomatic. The mortality rate at this stage is approximately 2% to 3%. Only 30% of those infected demonstrate overt signs and symptoms of the protracted disease affecting the heart (50%), esophagus, and colon (12%). Any other organ or system may be affected, but to a lesser extent.
In the initial stage, diagnosis is made by direct examination of a thin blood sample and identification of the parasites. Cure can be obtained at this stage with the use of drugs. In the chronic stage, no medication is helpful.
Symptomatic Esophageal Involvement
The main symptoms include dysphagia, pain, regurgitation, singultus, salivation with hypertrophy of the salivary gland, cough, constipation, and weight loss. Difficulty in swallowing is seen in most patients with Chagas’ megaesophagus; this may appear suddenly or slowly. The latter circumstance is most common and frequently follows a hurried meal containing solid food or occurs after an emotional episode. Cold food is not well tolerated. Patients learn several maneuvers to get food down into the stomach, such as arching backward, swallowing air, drinking large amounts of liquids, or taking deep breaths. As the dysphagia worsens, these patients are no longer able to eat a decent meal among unaffected people.
The pain has two manifestations. Odynophagia is seen at the time of swallowing and almost always means a hyperactive esophagus in the early stages of the disease. Pain radiates to the jaws, shoulder, and arm, lasting from a few minutes to hours. It is not related to reflux and is relieved with the use of sublingual nitrates or surgical treatment. The second type of pain or discomfort is described as retrosternal or substernal. Substernal pain may be elicited after eating a solid meal rapidly and is relieved by self-induced regurgitation. This maneuver is soon learned and is frequently used to relieve the symptoms. Food impaction causes constant pain, requiring endoscopic removal.
Regurgitation is a frequent symptom, with the contents depending on the stage of disease. In the early phase, regurgitation occurs soon after eating; the material regurgitated is clear and contains saliva and very little food. In the advanced stage, regurgitation is delayed and frequently occurs during the night. The regurgitated material contains undigested fermented food. At night, the patient is frequently awakened with cough and the sensation of suffocating. This symptom occurs in 55% to 90% of cases and should be distinguished from vomiting, which is unusual in megaesophagus.
Singultus (hiccup) may be an early indication of esophageal involvement; it may cause disabling discomfort lasting for days or weeks, requiring urgent treatment. The heartburn sensation frequent in gastroesophageal reflux (GER) is uncommon in megaesophagus. Excessive salivation and hypertrophy of the salivary glands, especially involving the parotid glands, are common findings in Chagas’ megaesophagus.
In the more advanced stage, cough is seen in more than 20% of the patients and usually occurs at night because of aspiration of esophageal contents into the pharynx and trachea. Three percent to 5% of patients present with pulmonary complications of an inflammatory nature.
Bowel constipation is attributable to two mechanisms. The first is that food is retained in the esophagus and little feces are formed; the second is associated megacolon. Constipation occurs in 22% of cases in our61 experience. Under these circumstances, the megaesophagus should be treated first to relieve constipation and dysphagia.
Malnutrition is common and is usually seen in younger patients. Regurgitation is frequent and leads to emaciation and cachexia. In children, lack of normal physical growth and mental retardation are seen. The triple A syndrome (adrenal insufficiency, achalasia of the cardia, and alacrima), is usually seen in childhood and was described by Allgrove and associates1 in 1978. This is a rare autosomal recessive condition. The AAAS gene product is a protein named ALADIN (alacrima, achalasia, adrenal insufficiency, neurologic disorder), which comprises 546 amino acids and belongs to the WD repeat family. Neuromuscular abnormalities, as noted by Orrell and Clark,34 may also be associated with this syndrome, with slowly progressive distal muscular atrophy in the legs, dysmorphic facial features, and moderate mental retardation.
Diagnosis
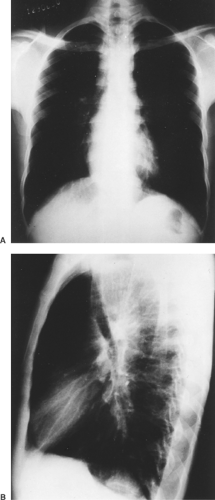
Chagas’ megaesophagus is strongly suspected on clinical grounds alone and is diagnosed by radiologic examination, scintigraphy, endoscopy, manometry, and laboratory tests. Plain chest radiographs may demonstrate some features usually seen in Chagas’ megaesophagus, such as an air–fluid level, tracheal deviation, mediastinal enlargement, lung changes, and absent gastric air bubble (Fig. 150-2).
Radiologic Studies
Before proceeding with contrast-enhanced studies of the esophagus, one should take time to empty the organ with a large-bore tube to facilitate visualization of any changes that may be present. The same should be done before endoscopy and surgery. The patient should be examined in both supine and upright positions to evaluate esophageal motility, stasis, the possible presence of esophageal carcinoma, and opening of the lower esophageal sphincter (LES).
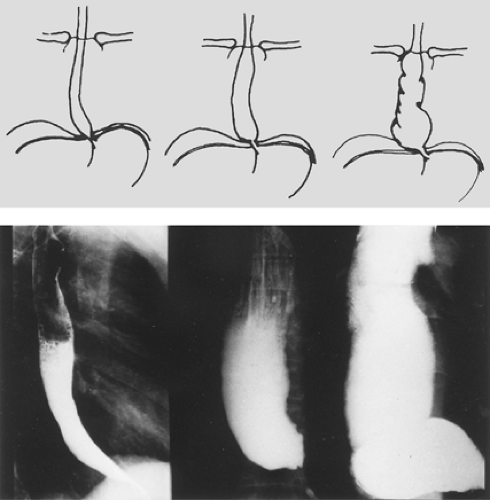
Based on the findings of contrast retention, caliber, contractility, tonicity of lower segment, size, and transverse diameter of the organ, Zucoloto and Rezende64 divided the chagasic mega- esophagus into three stages. In stage I, the esophagus is not dilated but the barium meal is partially retained and an air column remains along the length of the organ. The transverse diameter is up to 4 cm, and stasis is noted at 10 seconds. In stage II, mild
dilatation and uncoordinated muscular activity are noted in the lower part of the esophagus. The transverse diameter is between 4 and 7 cm, and barium stasis occurs after 5 minutes. In stage III, the esophagus is hypotonic and hypokinetic, with dilatation and stasis after 30 minutes. The transverse diameter is beyond 7 cm, and it is sometimes seen to lie down over the diaphragm (the so-called sigmoid esophagus) (Fig. 150-3). Staging of the disease process is important when it comes to deciding on treatment.
dilatation and uncoordinated muscular activity are noted in the lower part of the esophagus. The transverse diameter is between 4 and 7 cm, and barium stasis occurs after 5 minutes. In stage III, the esophagus is hypotonic and hypokinetic, with dilatation and stasis after 30 minutes. The transverse diameter is beyond 7 cm, and it is sometimes seen to lie down over the diaphragm (the so-called sigmoid esophagus) (Fig. 150-3). Staging of the disease process is important when it comes to deciding on treatment.
The radiologic examination is performed in the following manner. The patient fasts for 12 hours. After the esophagus is emptied, the barium meal (200 mL of water and 100 g of barium) is delivered, and three exposures are obtained at 180-cm distance within 10 seconds, 5 minutes, and 30 minutes after the barium swallow.
Scintigraphy
Radioisotope scanning has been used extensively to verify esophageal transit time and dysfunction and to assess the therapeutic response to treatment, including the presence of GER disease (GERD). The test is performed in the supine and sitting positions after the patient has swallowed a liquid or solid meal containing technetium-99m (99mTc) sulfur colloid (100–300 mL). The counts are taken in the area of interest and at fixed intervals (every 0.25 second for 40 seconds) and between the upper third, middle, and lower third of the esophagus and stomach.
With this technique, Rezende45 found a normal esophageal transit time of 8.3 ± 2.2 seconds (mean ± standard deviation) in the supine position and 6.3 ± 1.1 seconds in the sitting position. When the transit time exceeded 40 seconds, two patterns were observed: partial retention and total retention. Cases where the shuttling movement of the radioisotope between the distal and proximal esophagus had an uncoordinated transit pattern were classified as adynamic. This method agreed with manometric findings in 88.6% of the cases and had a sensitivity of 86.3% and a specificity of 93.3%. In summary, Rezende45 found the following transit patterns: normal, prolonged, partial retention (with and without incoordination), and total retention with incoordination (adynamic). Patients with peristaltic activity in the esophagus had a normal esophageal transit time in the supine position and some retention while in the erect position regardless of the function of the LES. Those without peristaltic waves had retention in both positions, whether the lower sphincter was functioning or not.
Endoscopy
Endoscopic examination should be performed before any attempt is made to treat megaesophagus surgically. The organ should be thoroughly washed to verify all possible changes in the esophagus and stomach. The flexible scope is preferable to the rigid device. Rezende and associates45 performed 722 endoscopies in 600 patients with megaesophagus over a 7-year period. Of this total, 480 were untreated and 120 had been treated. The findings included the following: esophagitis, 15 cases (2.5%); reflux esophagitis, 41 (6.5%); esophageal stenosis, 8 (1.3%); cancer, 5 (0.8%); hiatal hernia, 3 (0.5%); esophageal varices,
2 (0.3%); and leukoplakia, 1 (0.2%). In the stomach, duodenal bile reflux was found in 173 patients (30.4%), chronic gastritis in 109 (18.2%), gastric ulcer in 10 (1.8%), gastric polyposis in 2 (0.4%), gastric cancer in 1 (0.2%), megabulb-dilated duodenum in 9 (1.6%), and duodenal ulcer disease in 10 (1.8%). The high incidence of duodenal bile reflux may be related to the denervation of that portion of the pylorus.
2 (0.3%); and leukoplakia, 1 (0.2%). In the stomach, duodenal bile reflux was found in 173 patients (30.4%), chronic gastritis in 109 (18.2%), gastric ulcer in 10 (1.8%), gastric polyposis in 2 (0.4%), gastric cancer in 1 (0.2%), megabulb-dilated duodenum in 9 (1.6%), and duodenal ulcer disease in 10 (1.8%). The high incidence of duodenal bile reflux may be related to the denervation of that portion of the pylorus.
Manometric Studies
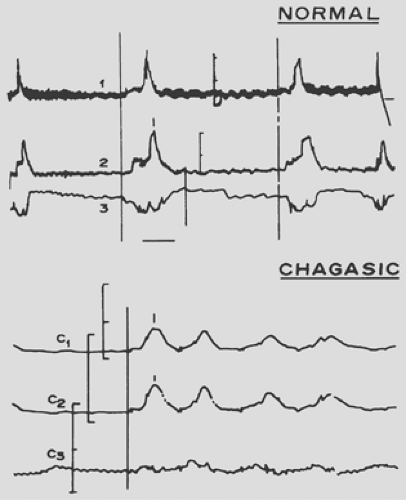
The depopulation of the ganglions in the chagasic esophagus leads to lack of peristalsis, achalasia of the LES, elevation of the resting pressure, and increased response to stimulation of the esophageal smooth muscle by cholinergic drugs. Swallowing in the normal person involves peristaltic and uncoordinated contractions with opening of the lower sphincter. In the chagasic individual, normal peristalsis is replaced by synchronous movement at all levels of the organ, and the LES does not open (Fig. 150-4). These alterations are observed in the lower third of the esophagus. The upper esophageal sphincter shows no changes, but the lower sphincter demonstrates lack of relaxation in approximately half of the patients in whom the esophagus is not dilated. In the more advanced cases with dilatation, the lower sphincter does not relax in more than 80% of patients.
Controversy surrounds LES pressure in chagasic individuals compared with those with idiopathic achalasia. According to Dantas and associates,14 some evidence shows that the denervation affects both inhibitory and cholinergic excitatory neurons. If this is true, it should be anticipated that the resting lower sphincter pressure will be low or normal in patients with Chagas’ disease compared with the elevated pressure in those with idiopathic achalasia. These differences may be related to the various methods of investigation.
Table 150-1 Diagnostic Methods in Chagas’ Disease | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree