Catheter Ablation Therapy for Arrhythmias
David E. Haines
Overview
The rationale behind ablative therapy is that, for any arrhythmia, a critical anatomic substrate allows propagation of that arrhythmia. If that substrate is irreversibly damaged or destroyed, then the arrhythmia should no longer occur spontaneously or with provocation.
Numerous methods of catheter ablation have been attempted experimentally and clinically, but radiofrequency catheter ablation is still accepted as the safest and most effective modality. Radiofrequency energy heats myocardium by the passage of the radiofrequency electrical current through the tissue, with consequent resistive tissue heating. Deeper tissue heating results in larger lesions. Larger electrodes, higher temperatures, and very high-power deliveries coupled with irrigated-tip or cooled-tip catheters will increase lesion size. Radiofrequency ablative lesions are thermally mediated. Temperatures exceeding 50°C result in breakdown of the sarcolemmal membrane and cell death.
The mechanisms of paroxysmal supraventricular tachycardia (SVT) are varied and include atrioventricular (AV) reciprocating tachycardia, AV nodal reentrant tachycardia, atrial fibrillation (AF), atrial flutter, and atrial tachycardia of focal automatic or reentrant mechanisms. Catheter ablation of accessory pathways responsible for the Wolff-Parkinson-White syndrome and AV reciprocating tachycardias may be accomplished at either the atrial or the ventricular insertion sites of the pathway, with a very high (>95% at experienced centers) success rate. Catheter modification of the AV node is highly successful in eliminating AV nodal reentrant tachycardia. In most cases, ablation in the low septal region results in elimination or marked modification of the slow AV nodal pathway and prevents tachycardia recurrence.
Atrial flutter is a macroreentrant rhythm that travels around the tricuspid valve annulus and through the isthmus between the valve and the inferior vena caval inlet. A linear ablative lesion placed across this isthmus results in successful ablation of the atrial flutter. AF may have a reentrant mechanism or, particularly in patients with frequent paroxysmal AF and normal atrial size, may originate from rapid firing from a pulmonary vein focus. Ablation of focal origins of AF initiation is relatively successful in eliminating this arrhythmia in highly selected patients. Combinations of pulmonary vein isolation, linear atrial ablation, atrial substrate modification, and atrial denervation have been employed with varying success as curative therapy for paroxysmal or persistent AF in patients with structural heart disease.
Ventricular tachycardia (VT) may be idiopathic or may result from the presence of underlying structural heart disease. Idiopathic VT from the outflow tract (most often right ventricle) is usually focal in origin, with a likely mechanism of abnormal automaticity or triggered activity. It may be ablated with a high likelihood of success. Idiopathic left VT is usually caused by reentry involving the fascicular network and may be cured with ablation of the fascicular insertion into the myocardium. VT in the setting of structural heart disease is usually the result of a reentrant mechanism in regions of patchy fibrosis. Ablation may be accomplished at sites within the zone of slowed electrical conduction that is identified with techniques such as activation and entrainment mapping.
Glossary
Accessory pathway
This anomalous bridge of electrically conducting tissue between the atrium and ventricle, which is responsible for Wolff-Parkinson-White syndrome and AV reciprocating tachycardias, is also known as a bundle of Kent or bypass tract.
Activation mapping
Multipoint electrogram acquisition during ongoing tachycardia demonstrates sites where the local
electrical activity precedes the onset of the surface QRS complex. These sites represent exit points from the slow conduction zone (SCZ) for reentrant tachycardias or sites of arrhythmia origin for automatic or triggered tachycardias.
electrical activity precedes the onset of the surface QRS complex. These sites represent exit points from the slow conduction zone (SCZ) for reentrant tachycardias or sites of arrhythmia origin for automatic or triggered tachycardias.
AV nodal modification
This consists of (a) ablation of the slow AV nodal pathway to treat AV nodal reentrant tachycardia and (b) nonspecific ablation of the AV node to impair anterograde conduction and to decrease the ventricular response rate to AF.
Coagulum
When boiling occurs at the electrode tip, coagulated protein from tissue and blood adheres to the tip of the electrode.
Convective cooling
This is the process by which thermal energy is removed by circulating blood flow through the heart.
Dispersive electrode
This electrode with a large surface area is placed in contact with the patient’s skin (usually with electrically conductive gel) to complete the electrical circuit during ablation with radiofrequency electrical current.
Electrical impedance
The resistance and capacitance of the radiofrequency electrical circuit; this includes the electronic components, the conducting wires, the point of conduction between the ablation electrode and the heart, the tissue between the heart and the skin, and the junction between the skin and the dispersive electrode.
Entrainment mapping
Pacing slightly faster than ongoing reentrant VT from various sites in the ventricle; when one is in or near the SCZ, the morphology of the resulting complex matches the tachycardia morphology exactly, and the time from the stimulus to the QRS complex is prolonged (concealed entrainment).
Focal initiation or focal site of origin
Some specific arrhythmias originate from a point source and emanate in a centripetal fashion from that point. The mechanisms of focal tachycardias are probably abnormal automaticity of triggered activity. Typical examples are some types of AF and idiopathic VT.
Impedance rise
If boiling and coagulum adherence occur, the available surface area for radiofrequency current conduction decreases, resulting in a sudden increase in system impedance.
Pace mapping
During sinus rhythm, pacing is performed from a variety of ventricular sites. For VTs of focal origin, the electrocardiograms (ECGs) during pacing should be almost identical to those during tachycardia if the pacing site is in close proximity to the arrhythmia origin.
Radiofrequency energy
High-frequency alternating electrical current; when passed through a resistive medium, it creates heat.
Slow and fast AV nodal pathways
The AV node has multiple atrial inputs that have varying properties, including conduction time. In typical AV nodal reentrant tachycardia, electrical activation travels in an anterograde fashion down a slowly conducting AV nodal pathway, and in a retrograde fashion up a fast conducting pathway.
Tricuspid–inferior vena cava isthmus
This is the narrow anatomic region between the tricuspid annulus and the inferior vena caval orifice in the right atrium through which atrial flutter reentrant circuits conduct. It is the usual site targeted for atrial flutter ablation.
Ventricular preexcitation
This pattern, seen on surface ECGs, is characterized by δ waves (slurred QRS upstrokes) and results from the presence of an accessory pathway that conducts in an anterograde fashion. Wolff-Parkinson-White syndrome is the most common form of preexcitation syndrome.
Ventricular tachycardia slow conduction zone (VT SCZ)
This region in the border zone of scar in the ventricle is bounded by areas of anatomic or physiologic conduction block, which has a slow conduction velocity. Conduction through this region accounts for the diastolic period of electrical silence on the surface ECG during ongoing reentrant VT.
Volume heating
This form of heating from radiofrequency energy or another energy source is direct and is not a result of heat conduction from a contiguous region of heated tissue.
General Principles
Ablative therapy for the management of arrhythmias is based on the observation that most arrhythmias arise from a focal origin or are critically dependent on conduction through a defined anatomic structure. If those critical regions are irreversibly damaged or destroyed, then the arrhythmia should no longer occur spontaneously or with provocation. Traditionally, ablation was performed with open surgical techniques. It was discovered, however, that focal endocardial injury could also be achieved by a controlled delivery of destructive energy through a catheter. Since the early 1990s, catheter designs have evolved to improve site access dramatically, and our knowledge and understanding of the anatomy of the heart and the sources of arrhythmia origin have significantly expanded. In response to this progress, the indications for catheter ablation have continued to broaden, and it is now the therapy of choice for a number of arrhythmias.
History of Catheter Ablation
The concept of ablative therapy for the treatment of arrhythmias was well established in the surgical literature throughout the 1970s. Since the original description of the surgical division of an accessory pathway by Sealey in 1969 (1), there had been several reports of surgical treatment of the Wolff-Parkinson-White syndrome (2,3) and VT (4,5). In these series, the concept of curative ablative therapy was proved. However, the obvious limitation to this approach was that open thoracotomy and, in most cases, cardiopulmonary bypass were required. Although researchers were pursuing a variety of approaches to decrease surgical morbidity and mortality, it was not thought possible to eliminate these arrhythmic substrates without direct visualization.
In 1979, a complication of transthoracic cardioversion was reported. Catheters had been placed for recording in the bundle of His position, and multiple direct current (DC) shocks were administered transthoracically to terminate an episode of VT. At the conclusion of the cardioversion, complete heart block was observed. It was hypothesized that shunting of defibrillator current through the His bundle catheter caused irreversible damage to the AV conduction system (6). After this report, Scheinman et al. (7) and Gallagher et al. (8) published the first series of catheter ablation of the AV junction in patients with AF and a rapid ventricular response rate that could not be controlled with drugs. In these patients, high-voltage DC electrical energy was intentionally delivered between the tip of an electrode catheter placed contiguous to the bundle of His, and a dispersive patch electrode was placed on the skin. The field of catheter ablation was born. Subsequently, investigators reported successful catheter ablation of accessory pathways (9,10) and VT (11) with this approach. Unfortunately, a relatively high complication rate was also reported, including pericardial tamponade, cardiogenic shock, and death (12).
The technique of high-energy DC shock catheter ablation was effective, but it was extremely difficult to control. An alternative energy source for controlled ablation of myocardium was needed. In 1985, Huang and associates described their
use of radiofrequency electrical energy for controlled catheter ablation of the AV junction in dogs. Radiofrequency catheter ablation resulted in very controllable, well-circumscribed endocardial lesions (13). It was discovered that lesion formation could be nicely controlled by either titrating power (14) or temperature measured at the catheter tip (15). Efficacy of radiofrequency catheter ablation in the clinical setting was increased with improvements in the catheter design. Larger ablation electrodes increased lesion size (16), and an increase in the electrode tip size from 2 to 4 mm increased procedure efficacy (17). Catheter design pioneers such as Will Webster constructed catheters that had a distal segment that could be deflected by manipulation of the control handle. This advance greatly improved site access and catheter stability.
use of radiofrequency electrical energy for controlled catheter ablation of the AV junction in dogs. Radiofrequency catheter ablation resulted in very controllable, well-circumscribed endocardial lesions (13). It was discovered that lesion formation could be nicely controlled by either titrating power (14) or temperature measured at the catheter tip (15). Efficacy of radiofrequency catheter ablation in the clinical setting was increased with improvements in the catheter design. Larger ablation electrodes increased lesion size (16), and an increase in the electrode tip size from 2 to 4 mm increased procedure efficacy (17). Catheter design pioneers such as Will Webster constructed catheters that had a distal segment that could be deflected by manipulation of the control handle. This advance greatly improved site access and catheter stability.
The first report of successful catheter ablation of an accessory pathway with high-frequency alternating (radiofrequency) electrical current by Borggrefe et al. brought us into the modern era of radiofrequency catheter ablation (18). Based on the important work of Jackman, Kuck, Haissaguerre, Morady, and others, the correlations among electrogram patterns, anatomic locations of accessory pathways, and access to those sites with ablation catheters were elucidated (19,20,21). Success rates exceeding 90% were reported by investigators in the treatment of paroxysmal SVT (22,23,24). In the present day, experienced operators achieve greater than 95% success rates in the ablation of these arrhythmias. The indications for radiofrequency catheter ablation continue to expand, and it has become a dominant therapeutic modality for many varieties of symptomatic tachycardia.
Biophysics of Radiofrequency Catheter Ablation
Radiofrequency electrical energy is employed to create thermal lesions in the heart. The frequencies generally employed are 300 to 1,000 kHz. Although this energy is similar to that employed for broadcast radio, the radiofrequency energy is electrically conducted, not radiated, during catheter ablation. The radiofrequency current is similar to low-frequency alternating current or DC with regard to its ability to heat tissue and create a lesion, but it oscillates so rapidly that cardiac and skeletal muscles are not stimulated, thereby avoiding induction of arrhythmias and decreasing the pain perceived by the patient. Electrical energy dissipates as heat within the first 2 mm of the tissue (direct resistive or volume heating) (15). Heating to deeper tissue layers occurs by heat conduction from the region of volume heating.
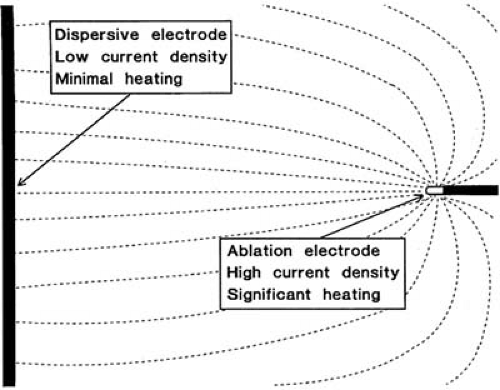
The radiofrequency current is generally delivered in a unipolar fashion between the tip of the ablation electrode and a dispersive electrode applied to the patient’s skin. Because the surface area of the ablation electrode is much smaller than that of the dispersive electrode, the current density is higher at the ablation site, and heating occurs preferentially at that site (Fig. 73.1). If, however, ablation is performed with a high-amplitude current, and skin contact by the dispersive electrode is poor, it is possible to cause skin burns (25). Nath et al. (26) demonstrated that the position of the dispersive electrode has little effect on the geometry of the resulting lesion. However, sometimes it is advantageous to increase the surface area of the dispersive electrode, particularly if the ablation is power limited. With lower impedance at the dispersive electrode-skin interface, a greater proportion of the available electrical energy will be available for heating at the tip of the ablation catheter. In patients who had a baseline system impedance of greater than 100 ohm, using two dispersive electrodes resulted in more effective heating than using a single electrode.
It has been demonstrated in vitro that, at steady state, the tissue temperature decreases radially in proportion to the distance from the ablation electrode and that, at steady state, the lesion size is proportional to the temperature measured at the interface between the tissue and the electrode (14). In addition, lesion size has also been shown to be proportional to the radiofrequency power amplitude. Using higher powers and achieving higher tissue temperatures may increase lesion size. However, once the peak tissue temperature exceeds the threshold of 100°C, boiling at the electrode-tissue interface may ensue (27). When boiling occurs, denatured serum proteins and charred tissue adhere to the electrode to form an electrically insulating “coagulum,” which is accompanied by a sudden rise in electrical impedance that prevents further current flow into the tissue and further heating. The consequences of this adverse event could include char embolism or excess disruption of the endocardium with subsequent thrombus formation and associated risk of thromboembolism. Conventional electrode catheters with temperature monitoring underestimate peak tissue temperature, so it is best if target temperatures not exceeding 70°C to 80°C are selected in the clinical setting. Because the rate of temperature rise at deeper sites within the myocardium is slow (28), a continuous energy delivery of at least 60 seconds is often warranted to maximize depth of lesion formation. Tissue temperatures continue to rise for several seconds after termination of radiofrequency energy delivery (29). This thermal latency effect can account for the observation that patients undergoing AV modification procedures and demonstrating transient heart block during radiofrequency energy delivery may progress to persisting complete heart block even if radiofrequency energy delivery is terminated immediately.
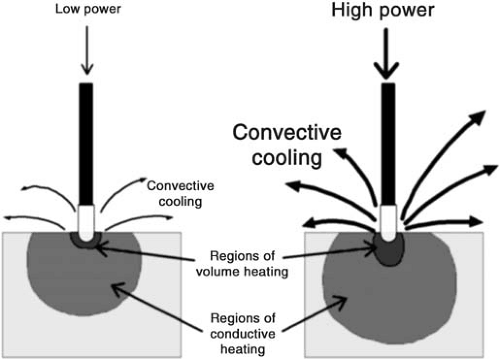
The dominant factor opposing effective heating of myocardium is the convective cooling from the circulating blood pool. If the catheter position is not stable, or if it is positioned in region of high blood flow, the magnitude of convective cooling is increased (30). Efficiency of energy delivery to the tissue may vary from approximately 70% to 10% in vivo. The effects of convective cooling have been exploited to increase the size of catheter ablative lesions. To eliminate the risk of
overheating at the electrode-tissue contact point but increase the magnitude of power delivery and the depth of volume heating, investigators have employed porous-tipped electrodes for open irrigation of the electrode tip or closed irrigation systems for electrode tip cooling (Fig. 73.2). Nakagawa et al. achieved temperatures of 95±9°C at depths of 3.5 mm and lesion dimensions of 10±1 mm depth by 14±2 mm diameter with ablation through an irrigated-tip electrode in a superfused canine thigh muscle preparation. However, superheating within the tissue with a resulting sudden explosive release of the expanding steam to the surface (the so-called pop lesion) was observed in 7.5% of the ablations (31). Cooled-tip catheters have now been used extensively in the clinical setting. The larger lesions generated have yielded increased procedure success rates or decreased procedure times, particularly with ablation of difficult substrates such as atrial flutter (32,33).
overheating at the electrode-tissue contact point but increase the magnitude of power delivery and the depth of volume heating, investigators have employed porous-tipped electrodes for open irrigation of the electrode tip or closed irrigation systems for electrode tip cooling (Fig. 73.2). Nakagawa et al. achieved temperatures of 95±9°C at depths of 3.5 mm and lesion dimensions of 10±1 mm depth by 14±2 mm diameter with ablation through an irrigated-tip electrode in a superfused canine thigh muscle preparation. However, superheating within the tissue with a resulting sudden explosive release of the expanding steam to the surface (the so-called pop lesion) was observed in 7.5% of the ablations (31). Cooled-tip catheters have now been used extensively in the clinical setting. The larger lesions generated have yielded increased procedure success rates or decreased procedure times, particularly with ablation of difficult substrates such as atrial flutter (32,33).
Because the success of radiofrequency catheter ablation in the clinical setting is sometimes limited by the relatively small size of the lesion, attempts have been made to increase the size of those lesions reliably and safely. One approach toward this end is to increase the size and surface area of the electrode. The radiofrequency power needs to be increased comparably to achieve a similar current density and temperature at the electrode-tissue interface, and the result is a greater depth of volume heating and a larger lesion (16). Conventional ablation catheters employ 4- and 5-mm electrode tips, but catheters with 8 and 10-mm tips are now being used for difficult ablation sites where larger lesion size and depth are required (34). It is not clear that one large lesion technology is better than another (35), but each method has its advantages and disadvantages (36).
Mechanisms of Myocardial Injury Caused by Radiofrequency Catheter Ablation
It is likely that the major mechanism of tissue injury from radiofrequency catheter ablation is thermal. Heating of the myocardium occurs reproducibly during radiofrequency energy delivery, and the association of clinical effect and temperature measured at the electrode catheter interface has been established. Experimentally, lesions created from a radiofrequency or microwave source have a reliable isotherm of irreversible tissue injury of 52°C to 55°C (37). In the clinical setting, successful ablations were associated with a mean temperature measured at the electrode-tissue interface of 62±15°C (38). During ablation of the AV junction, the reversible physiologic effect of an accelerated junctional rhythm was observed at temperatures of 51±4°C, whereas temperatures of 58±6°C were required to achieve heart block (39). These findings are consistent with an anatomic position of the ablation target that may several millimeters from the electrode-tissue contact point.
Tissue Effects of Radiofrequency Catheter Ablation
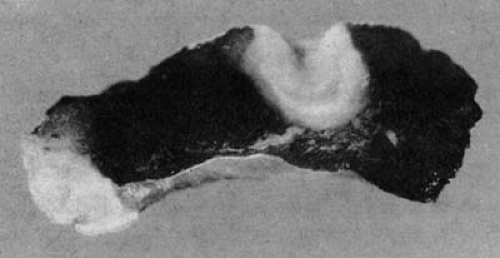
Changes in myocardial tissue are apparent immediately on completion of the radiofrequency lesion. Pallor of the central zone of the lesion is attributable to myoglobin denaturation with an associated color change. Some volume loss in the central region of lesion formation is apparent, as evidenced by a slight deformation at the point of catheter contact. Fibrin usually adheres to the endocardial surface, and if a temperature of 100°C has been exceeded, adherent char and thrombus are often apparent. On sectioning, the central portion of the lesion shows desiccation, with a surrounding region of hemorrhagic tissue, then normal-appearing tissue (Fig. 73.3) (40). Histologic examination of an acute lesion shows typical coagulation necrosis with basophilic stippling consistent with intracellular calcium overload. Immediately surrounding the central lesion is a region of hemorrhage and acute monocellular and neutrophilic inflammation. The progressive changes seen in the evolution of a radiofrequency lesion are typical of healing after any acute injury. Within 2 months of the ablation, the lesions show fibrosis, granulation tissue, chronic inflammatory infiltrates, and significant volume contraction (40). The lesion border is well demarcated from the surrounding viable myocardium without evidence of patchy fibrosis. This finding likely accounts for the absence of proarrhythmia side effects with radiofrequency catheter ablation. Because of the high-velocity blood flow within the epicardial coronary arteries,
these vessels are continuously cooled and are spared from injury despite nearby delivery of radiofrequency energy (41). However, high-power radiofrequency delivery in small hearts, such as in pediatric patients, may potentially cause coronary arterial injury, so caution is warranted (42).
these vessels are continuously cooled and are spared from injury despite nearby delivery of radiofrequency energy (41). However, high-power radiofrequency delivery in small hearts, such as in pediatric patients, may potentially cause coronary arterial injury, so caution is warranted (42).
The border zone around the acute pathologic lesion is a region that can demonstrate initial stunning and then early or late recovery of function, or it can show late progression of physiologic block, resulting in a “delayed cure” in some cases (43). Significant diminution in microvascular blood flow is observed in this region. Electron microscopic examination of the border zone has demonstrated marked ultrastructural abnormalities in tissue that appears to be viable by routine histologic examination. The microvessels appear severely damaged with loss of the basement membrane, disruption of the endothelial cell plasma membrane, and erythrocyte stasis (44). The myocytes show significant ultrastructural injury of the plasma membrane, mitochondria, sarcomeres, sarcoplasmic reticulum, and gap junctions. The most thermally sensitive structures appear to be the plasma membrane and gap junctions, which show morphologic changes as far as 6 mm from the edge of the histologic lesion (45). It has therefore been documented that the effects of radiofrequency lesion formation extend well beyond the acute pathologic lesion and are characterized by marked ultrastructural abnormalities of the microvascular and myocytes acutely and a typical inflammatory response later. Therefore, the recovery of electrophysiologic function after successful catheter ablation in the clinical setting may result from healing of the damaged but surviving myocardium. Moreover, the progression of the electrophysiologic effects after completion of the ablation procedure may result from further inflammatory injury and necrosis in the border zone region.
Cellular Effects of Radiofrequency Ablation
Hyperthermic injury to the cell is both time and temperature dependent. This injury to the myocyte may be caused by changes in the membrane, protein inactivation, cytoskeletal disruption, nuclear degeneration, or a number of other potential mechanisms. Experimentally, brief duration of exposure to heat results in prominent changes in the electrophysiology of the myocyte. In one study, guinea pig papillary muscles were exposed to temperatures between 37°C and 55°C, and transmembrane potentials were measured. In the low hyperthermic range (37°C to 45°C), a minor change was seen in the resting membrane potential and action potential amplitude, and the action potential duration shortened significantly. In the intermediate hyperthermic range (45°C to 50°C), progressive depolarization of the resting membrane potential, loss of action potential amplitude, abnormal automaticity, and reversible loss of excitability were seen. In the high temperature ranges (>50°C), marked depolarization of the resting membrane potential, permanent loss of excitability, and contracture of the preparation were observed (46). In a similar preparation, hyperthermia was shown to increase intracellular calcium significantly. Calcium entry into the cell was not channel specific and was buffered by uptake by the sarcoplasmic reticulum (47). Another study examined the effects of hyperthermia on conduction velocity of a preparation of epicardial shavings from canine left ventricular free walls. Conduction velocity was greater than at baseline between 38.5°C and 45.4°C, but at temperatures higher than 45.4°C, a progressive drop in conduction velocity was seen, followed by temporary (49.5°C to 51.5°C) then permanent (51.7°C to 54.4°C) conduction block (48). It is hypothesized that these electrophysiologic changes may be caused by nonspecific ion transit through thermally induced transmembrane pores. In particular, acute intracellular calcium overload may be the dominant mechanism of cellular contracture and death at temperatures higher than 50°C.
Ablation of Specific Supraventricular Arrhythmias
Accessory Pathway–Mediated Arrhythmias
A giant step forward was achieved with the first successful catheter ablations of accessory pathways (18). Before the catheter ablation era, patients with Wolff-Parkinson-White syndrome and concealed accessory pathways frequently required open surgical ablation of their extranodal pathways. The ability to map the location of accessory pathways precisely and to deliver a very precise ablative lesion with radiofrequency energy not only proved to be a valuable therapeutic modality, but also greatly enhanced the understanding of the physiologic-anatomic correlates of this relatively common abnormality. Important preliminary work by Jackman et al. identified electrogram patterns that correlated precisely with accessory pathway insertions in the atria and ventricles (19). The ultimate validation of the origin of these potentials arrived when small, discrete radiofrequency lesions placed at those locations resulted in elimination of both anterograde and retrograde accessory pathway conduction (Fig. 73.4) (22,23,24,49). Most accessory pathways have a discrete and narrow ventricular and atrial insertion, but they sometimes branch and often have a slanting course (19). The high success rate of radiofrequency catheter ablation delivered from the endocardial approach implies that most pathways are situated close to the endocardial surface. However, a small proportion of left free wall accessory pathways (1% to 4%) can be ablated successfully only from the epicardial approach via the coronary sinus and probably represent true epicardial pathways (50).
The successful catheter ablation of accessory pathways is directly related to the skill and experience of the operator (51). Careful mapping of the accessory pathway atrial and ventricular insertion points before any radiofrequency energy delivery greatly enhances the efficiency of the ablation procedure and minimizes the risk of distortion of local electrograms by poorly placed ablative lesions. In patients with manifest ventricular preexcitation (Wolff-Parkinson-White syndrome), mapping is best performed in the anterograde direction during sinus or
atrial-paced rhythm. The local ventricular activation recorded from the mapping/ablation catheter should precede the onset of the δ wave on the surface ECG by at least 10 to 20 milliseconds, and the electrogram pattern should be stable, indicating stable catheter-tissue contact (Fig. 73.5). An excellent ablation site will show the presence of a high-frequency accessory pathway potential immediately preceding the local ventricular activation and a short atrial to ventricular electrogram interval (52,53). When the accessory pathway is concealed (absent anterograde but intact retrograde conduction), mapping of retrograde activation during ventricular pacing or ongoing orthodromic AV reciprocating tachycardia must be pursued. Discrimination between retrograde AV nodal conduction and conduction up the accessory pathway may be enhanced by pacing near the pathway’s ventricular insertion site. The atrial insertion of the accessory pathway is determined by identifying the site with the shortest interval from the reference ventricular electrogram to the local atrial activation from the mapping/ablation electrode. One must be cautious not to mistake late components of the local ventricular electrogram for early atrial signals. As with anterograde mapping, the presence of a local high-frequency accessory pathway potential is an excellent marker for successful ablation sites.
atrial-paced rhythm. The local ventricular activation recorded from the mapping/ablation catheter should precede the onset of the δ wave on the surface ECG by at least 10 to 20 milliseconds, and the electrogram pattern should be stable, indicating stable catheter-tissue contact (Fig. 73.5). An excellent ablation site will show the presence of a high-frequency accessory pathway potential immediately preceding the local ventricular activation and a short atrial to ventricular electrogram interval (52,53). When the accessory pathway is concealed (absent anterograde but intact retrograde conduction), mapping of retrograde activation during ventricular pacing or ongoing orthodromic AV reciprocating tachycardia must be pursued. Discrimination between retrograde AV nodal conduction and conduction up the accessory pathway may be enhanced by pacing near the pathway’s ventricular insertion site. The atrial insertion of the accessory pathway is determined by identifying the site with the shortest interval from the reference ventricular electrogram to the local atrial activation from the mapping/ablation electrode. One must be cautious not to mistake late components of the local ventricular electrogram for early atrial signals. As with anterograde mapping, the presence of a local high-frequency accessory pathway potential is an excellent marker for successful ablation sites.
Using electrogram criteria as described earlier, Jackman and colleagues reported an initial accessory pathway catheter ablation success rate of 99% using a median of three radiofrequency energy deliveries (22). In a comparison of the electrograms from 49 successful versus 462 failed ablation sites, an interval of the local ventricular electrogram to δ-wave onset of greater than 10 milliseconds had a sensitivity of 98% for successful ablation, but only a positive predictive value of 11% (53). The low specificity of this finding may have been caused, in part, by inadequate tissue heating by the ablation catheter despite optimal site selection. The presence of ongoing AF can complicate and prolong mapping of accessory pathways because of the absence of consistent atrial electrograms and an inability to measure the AV times. However, if the same criteria of local ventricular electrogram to δ-wave interval and presence of accessory pathway potentials are employed, a high (95%) success rate can still be achieved (54). The characteristic of the local unipolar electrogram is also useful in identifying successful ablation sites. If the recording electrode is placed directly on the accessory pathway insertion site, all conduction into the ventricle (during anterograde mapping) or atrium (during retrograde mapping) will follow a vector away from the electrode. Thus, a “QS” unipolar electrogram pattern should be more favorable than an electrogram pattern with any initial positive deflection. The positive predictive value of this feature reviewing 186 separate ablation sites in 56 patients was 86% and 92% for ventricular and atrial mapping, respectively. The negative predictive value overall was 92% (55).
Catheter placement for ablation of accessory pathways on the left free wall may be accomplished by two approaches. The catheter may be passed across a small puncture hole in the fossa ovalis (transseptal) (56), or it may be prolapsed across the
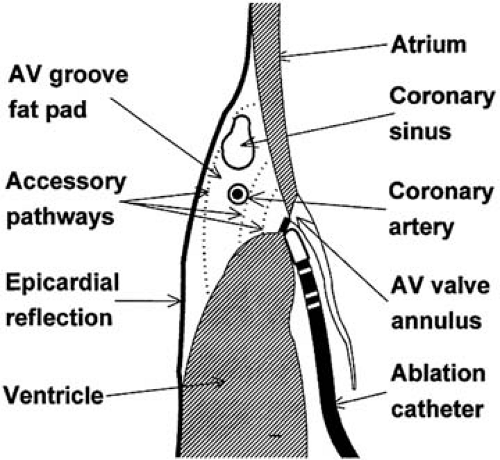
aortic valve and manipulated in the left ventricle back to the mitral annulus (retrograde) (22,23). Direct comparisons of the two techniques have yielded similar success rates (57), although in children and in patients older than 65 years, complications and failure rates may be higher with retrograde versus transseptal catheter placement (58). Catheter ablation of right free wall pathways is technically challenging and has a lower success rate than ablation of left free wall pathways (51), because it is difficult to stabilize the catheter position on the tricuspid annulus. Multiple accessory pathways are more frequently found in association with right free wall pathways than other locations (≤10% of cases). A subset of patients with Ebstein anomaly has a high prevalence of Wolff-Parkinson-White syndrome, often with multiple accessory pathways. Catheter ablation in this group of patients is made more difficult by the finding that the tricuspid valve (the normal anatomic landmark for catheter placement) is displaced downward from the true anatomic annulus where the accessory pathways are located. Septal accessory pathways may be divided into anteroseptal, midseptal, and posteroseptal locations. Anteroseptal and midseptal pathways are located in close contiguity to the normal AV conduction system. Radiofrequency catheter ablation in these region may be associated with a higher than normal risk of complete heart block (59,60). Use of cryothermic ablation in this region may improve catheter stability and may be safer overall (61). The posteroseptal space is a complex anatomic region characterized by a broad region where the AV valve annuli are offset and diverge, resulting in a region of fatty and connective tissue that is not immediately accessible from endocardial ablation electrode positions and frequently requires ablation from within the coronary sinus (22,50,62). The anatomic courses of slowly and decrementally conducting accessory pathways that activate the ventricle with a left bundle branch block configuration (also known as Mahaim pathways) have been demonstrated commonly to originate from the right atrial free wall. Discrete pathway potentials may be mapped from the tricuspid annulus, along the right ventricular endocardial surface to arborized insertions into the right bundle branch. Application of radiofrequency energy along this course successfully ablates the pathways and prevents further AV reciprocating tachycardia (63,64,65).
aortic valve and manipulated in the left ventricle back to the mitral annulus (retrograde) (22,23). Direct comparisons of the two techniques have yielded similar success rates (57), although in children and in patients older than 65 years, complications and failure rates may be higher with retrograde versus transseptal catheter placement (58). Catheter ablation of right free wall pathways is technically challenging and has a lower success rate than ablation of left free wall pathways (51), because it is difficult to stabilize the catheter position on the tricuspid annulus. Multiple accessory pathways are more frequently found in association with right free wall pathways than other locations (≤10% of cases). A subset of patients with Ebstein anomaly has a high prevalence of Wolff-Parkinson-White syndrome, often with multiple accessory pathways. Catheter ablation in this group of patients is made more difficult by the finding that the tricuspid valve (the normal anatomic landmark for catheter placement) is displaced downward from the true anatomic annulus where the accessory pathways are located. Septal accessory pathways may be divided into anteroseptal, midseptal, and posteroseptal locations. Anteroseptal and midseptal pathways are located in close contiguity to the normal AV conduction system. Radiofrequency catheter ablation in these region may be associated with a higher than normal risk of complete heart block (59,60). Use of cryothermic ablation in this region may improve catheter stability and may be safer overall (61). The posteroseptal space is a complex anatomic region characterized by a broad region where the AV valve annuli are offset and diverge, resulting in a region of fatty and connective tissue that is not immediately accessible from endocardial ablation electrode positions and frequently requires ablation from within the coronary sinus (22,50,62). The anatomic courses of slowly and decrementally conducting accessory pathways that activate the ventricle with a left bundle branch block configuration (also known as Mahaim pathways) have been demonstrated commonly to originate from the right atrial free wall. Discrete pathway potentials may be mapped from the tricuspid annulus, along the right ventricular endocardial surface to arborized insertions into the right bundle branch. Application of radiofrequency energy along this course successfully ablates the pathways and prevents further AV reciprocating tachycardia (63,64,65).
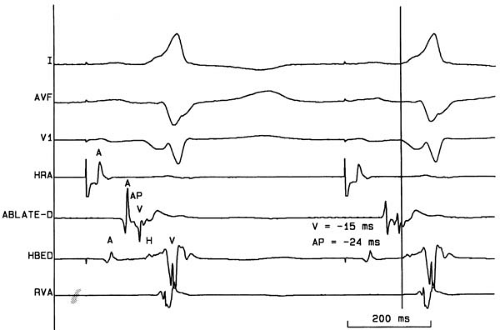 FIGURE 73.5. Surface electrocardiogram tracings I, aVF, and V1 and intracardiac recordings from the high right atrium (HRA), distal ablation catheter bipole (ABLATE-D), the distal His bundle bipole (HBED), and the right ventricular apex (RVA) in a patient with the Wolff-Parkinson-White syndrome. The vertical line
Get Clinical Tree app for offline access
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree


|