Abnormal impulse initiation
•Automaticity
–Enhanced normal automaticity: as seen in inappropriate sinus tachycardia.
–Abnormal automaticity: as in ectopic atrial tachycardia, accelerated junctional rhythm, and idiopathic ventricular tachycardias.
•Triggered activity
–Early after-depolarization: implicated in Torsades de pointes in long QT syndromes.
–Delayed after-depolarization: seen in digitalis-induced arrhythmias and paroxysmal catecholaminergic ventricular tachycardia (CPVT).
Abnormal impulse conduction |
|---|
•Anatomical reentry: |
–Related to normal anatomic boundaries: typical atrial flutter. –Related to acquired boundaries: ventricular tachycardia late after myocardial infarction or surgical ventricular incisions, atrial tachycardias related to surgical atrial incisions. –Random reentry and rotors: as seen in atrial fibrillation. |
•Other concepts (reflection, functional reentry, and anisotropic reentry). |
28.3 Clinical Presentation and Diagnosis
Arrhythmias can and often do occur in an apparently normal heart [e.g., premature atrial or ventricular beats, paroxysmal supraventricular tachycardias (PSVTs)]. However, other clinically important rhythm disturbances are more commonly associated with genetic or structural heart disease. Today, myocardial ischemia is the most important substrate for serious arrhythmias in the Western world, but other forms of nonischemic cardiomyopathy, valvular heart disease, and genetically determined disorders (e.g., long QT syndrome, Brugada syndrome) are also culprits. In Western countries, viral and rare bacterial infections such as Lyme disease can cause myocarditis, and can be sources of serious arrhythmias. In other parts of the world, infections such as rheumatic heart disease in Africa and Asia, or Chagas disease in South America, remain as important underlying causes of heart rhythm disturbances.
Importantly, the clinical presentation of cardiac arrhythmias may range widely from being completely asymptomatic or mildly symptomatic (palpitations and anxiety) to syncope and even sudden cardiac death (SCD); the clinical impact is largely dependent on arrhythmia-induced hemodynamic changes. The electrophysiologic and hemodynamic consequences of a particular arrhythmia are primarily determined by the: (1) ventricular rate and duration; (2) site of origin; (3) underlying cardiovascular status (i.e., severity of associated heart and vascular disease); and/or (4) promptness and completeness of autonomic nervous system responses to the arrhythmia-induced “stress.”
Some arrhythmias can be readily diagnosed by standard 12-lead electrocardiography (ECG), but in other patients its use is often too brief to detect transient rhythm problems. Consequently, other techniques are frequently required to establish an accurate diagnosis including: (1) prolonged ambulatory ECG monitoring (e.g., event monitors, Holter monitors, or mobile outpatient cardiac telemetry); (2) implantable loop recorders; and/or (3) clinical electrophysiologic testing. In selected cases, additional exercise stress testing and signal-averaged ECG recordings may also be used to assess the general susceptibility of a given patient to arrhythmias. Other techniques have provided useful research information, but their value in daily practice remains to be fully defined at this time, such as: (1) analyses of heart rate variability; (2) relative baroreflex sensitivity; (3) assessment of QT dispersion or T-wave alternans; and/or (4) body surface potential mapping.
28.4 Treatment Considerations
The primary goals of the treatment for arrhythmias are twofold: (1) to alleviate symptoms and thus improve the given patient’s quality of life; and (2) to prolong survival. Still today, pharmacologic treatments are the initial choice for management of most cardiac arrhythmias, although, in recent years, implantable devices and ablation have become increasingly important, and in several cases the “first line” therapy. The principal pharmacologic effects of antiarrhythmic drugs can be found within the Vaughn–Williams classification (Table 28.2). A more comprehensive classification, termed the Sicilian Gambit, was introduced in 1991 [1]; this was based on a more comprehensive consideration of the cellular basis of drug actions. In general, neither scheme is fully useful in the treatment of specific arrhythmias. Selection of antiarrhythmic drugs for a given patient should be individualized based on the arrhythmia being treated, the underlying heart disease and comorbidities, individualized responses, and potential side effects.
Table 28.2
Vaughn-Williams classificationa
Class I: Sodium channel blockers |
•Class Ia: drugs that reduce Vmax (phase 0 upstroke of action potential) and prolong action potential duration, such as quinidine, procainamide, and disopyramide. •Class Ib: drugs that do not reduce Vmax and shorten action potential duration, such as lidocaine, mexiletine, and phenytoin. •Class Ic: drugs that predominantly slow conduction, moderately reduce Vmax, and minimally prolong refractoriness, such as flecainide, propafenone, and moricizine. |
Class II: β–adrenergic receptor blockers |
•β-blockers may be cardio- or β1-selective such as atenolol, esmolol, and metoprolol or noncardioselective such as carvedilol, pindolol, and propranolol. •Some exert intrinsic sympathomimetic activity (acebutolol, bucindolol, and pindolol) •Some have quinidine-like membrane stabilizing activity (acebutolol, carvedilol, and propranolol). •d-sotalol has strong Class III effect and has been regarded as a Class III agent in many conditions. |
Class III: Potassium channel blockers that prolong refractoriness, such as amiodarone, bretylium, dofetilide, ibutilide, and sotalol. Amiodarone has all the four class effects. |
Class IV: Calcium channel blockers |
•Dihydropyridine (almodipine and nifedipine) •Nondihydropyridine drugs (diltiazem and verapamil). |
In patients with ischemia associated with left ventricular dysfunction, Class I antiarrhythmic agents should be avoided due to their proarrhythmic risks (as well as the tendency of this class to exhibit marked negative inotropic effects). Class III drugs, on the other hand (i.e., amiodarone, sotalol, and dofetilide), appear to elicit neutral effects on survival in these patients and have lesser negative inotropic concerns. Beta-blockers (Class II agents) have been shown to prolong survival in patients with structural heart disease. In terms of their use for suppression of symptomatic arrhythmias, beta-blockers are mainly useful for prevention of AV node-dependent reentrant supraventricular tachycardias (SVT) and for rate control of atrial fibrillation (AFib). Nevertheless, nonpharmacologic interventions, such as catheter ablation and implantable cardioverter defibrillator (ICD) therapy for primary and secondary prevention of SCD, are considered the treatment of choice in some clinical scenarios. For more details on pharmacologic effects on the heart, see Chaps. 26 and 30.
28.5 Tachyarrhythmias
28.5.1 Premature Complexes
Ectopic premature beats may originate from the atria, the AV junction, and/or the ventricles. Treatment of premature complexes is generally not necessary. In the symptomatic patient, precipitating factors (such as alcohol, tobacco, and caffeine) should be identified and eliminated. Although anxiolytic agents and beta-blockers may be tried, their efficacy is limited. Antiarrhythmic drugs may be used depending on the severity of symptoms and associated underlying cardiac disease, but avoidance of these drugs is the preferred strategy when possible.
28.5.1.1 Premature Atrial Complexes
Premature atrial complexes (PACs) are typically recognized on the ECG as early P-waves, with a P-wave morphology different from that of the sinus P-wave (Fig. 28.1a). Since the premature P-wave is often superimposed in the preceding T-wave, the T-wave preceding a premature beat should be carefully compared to other T-waves to identify any buried P–waves. PACs can conduct to the ventricles with a normal PR interval when the AV junction is not refractory, or with a prolonged PR interval when the AV junction is in its relative refractory period, or they can be blocked when the AV junction is in its effective refractory period. PACs almost always enter the sinus node and reset the sinus cycle length, resulting in an incomplete compensatory pause (the sum of pre- and post-PAC intervals is thus less than that of two normal sinus PP intervals). They are frequently found in normal subjects and are usually asymptomatic. Treatment is usually not necessary.
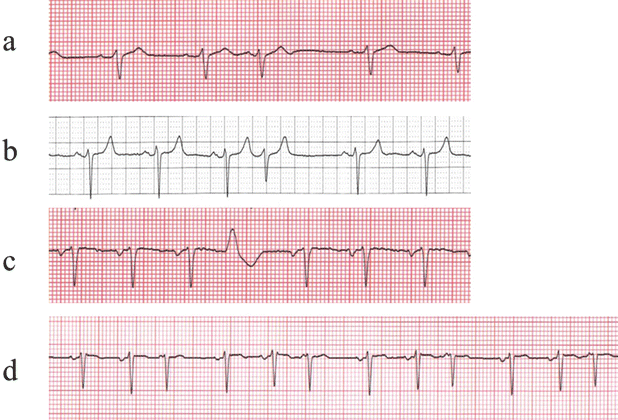

Fig. 28.1
Atrial (a), junctional (b), and ventricular (c) premature complexes, as well as multifocal atrial tachycardia (d)
28.5.1.2 Multifocal Atrial Tachycardias
Multifocal atrial tachycardia is a relatively uncommon arrhythmia characterized by atrial rates between 100 and 130 bpm with marked variations in P-wave morphology, arbitrarily defined as at least three different P-wave contours (Fig. 28.1d). Typically, multifocal atrial tachycardias occur in older patients with moderate to severe cardiopulmonary disease; they may be especially elicited during an exacerbation. Treatment in such cases is often difficult, and should be primarily directed toward the underlying lung disorder; typically calcium channel blockers are the treatment of choice. Beta-blockers and amiodarone can also be effective, but they are usually avoided due to underlying lung disease.
28.5.1.3 AV Junctional Premature Complexes
AV junctional premature complexes are typically recognized on the ECG as normal QRS complexes without a preceding P-wave (Fig. 28.1b). Further, retrograde P–waves (inverted in II, III, and aVF) may be seen after the QRS complexes. AV junctional complexes are less common than PACs and are often associated with drug intoxication and cardiac disease. Most junctional beats have an incomplete compensatory pause (like PACs) because they generate a retrograde atrial activation that resets the sinus node. On rare occasions, a junctional premature beat may fail to conduct to either the atria or ventricles (concealed junctional beats), but results in refractoriness in the AV junction and intermittent AV block.
28.5.1.4 Premature Ventricular Complexes
Premature ventricular complexes (PVCs) are recognized as wide QRS complexes of ventricular origin that are not preceded by P-waves (Fig. 28.1c). In addition, because they are premature they often fail to conduct retrograde to the atria. Thus, PVCs do not typically reset the sinus node, but they typically render the AV junction refractory to the subsequent sinus beat. The result is a full compensatory pause (the sum of pre- and post-PVC intervals equals that of two normal sinus PP intervals). On rare occasions, a PVC occurs between two consecutive normal sinus beats (interpolated PVC). In this case, the PVC neither resets the sinus node nor renders the AV node refractory, so the second sinus beat is conducted to the ventricle. PVCs may occur as a single event, but they also occur in patterns of bigeminy (sinus beat and PVC alternating) and trigeminy (two sinus beats followed by a PVC). Couplets or pairs (two consecutive PVCs) and nonsustained ventricular tachycardia (arbitrarily defined as 3 or more consecutive PVCs at a rate of >100 bpm) are also relatively common observations during the monitoring of patients with associated heart disease.
PVCs often bear a fixed coupling interval (between the onset of PVC and the onset of its preceding sinus QRS complex). When there is a protected ventricular ectopic focus, it is constantly firing without being reset by the preceding sinus beats; this is called ventricular parasystole which is characterized by varying coupling intervals. These intervals between the PVCs can be fixed or a multiplier of a common factor.
The relationship of PVCs to SCD is poorly defined. Frequent PVCs (>5–10 per minute) have been shown to be associated with increased risk of SCD. Recent data suggest that a high PVC burden could also be the etiology of left ventricular (LV) dysfunction. The challenge is to make sure to assess ejection fractions during the sinus rhythm beat in patients with very high burden. It should be noted that suppression of PVCs using antiarrhythmic drugs does not reduce, and may actually increase, the risk of SCD in patients following myocardial infarction [2, 3]. This indicates that mortality is primarily determined by the severity of underlying disease, and that the ectopy is an associated finding but not an independent determinant. Alternatively, any benefit derived from PVC suppression by conventional antiarrhythmic drugs may be counterbalanced by a drug-induced increment in mortality due to their negative inotropic and proarrhythmic effects.
Currently, intervention is indicated if the PVC: (1) becomes symptomatic; (2) is thought to be the source of LV dysfunction; or (3) is a trigger for more serious arrhythmias like ventricular tachycardia or ventricular fibrillation. In such cases, ablation therapy is often the preferred treatment since it has a relatively high success rate and can avoid prolonged medical therapies and their potential side effects.
28.5.2 Sinus Tachycardias
28.5.2.1 Physiological Sinus Tachycardias
Physiological sinus tachycardia represents a normal response to a variety of physiological (anxiety and exercise) and pathological stresses (fever, hypotension, thyrotoxicosis, hypoxemia, and congestive heart failure). Sinus tachycardia rarely exceeds 200 bpm. It should not be treated for itself, but its causes must be explored and some of these may require therapy (e.g., treatments of fever, anemia, or hyperthyroidism).
28.5.2.2 Inappropriate Sinus Tachycardias
Inappropriate sinus tachycardia (IST) is characterized by an increased resting heart rate (often >100 bpm) and an exaggerated heart rate response to minimal stress. Such a tachycardia is usually associated with distressing symptoms (palpitations, fatigue, anxiety, and/or shortness of breath). Unfortunately, their etiology and underlying mechanisms are often unclear.
Care must be taken in making a diagnosis of IST. Bona fide ISTs are rare, but it may be observed after previous cardiac arrhythmia ablation procedures or in the setting of symptoms suggestive of postural orthostatic tachycardia syndrome. When considering IST as a diagnosis, it is crucial to exclude secondary sinus tachycardias and to correlate symptoms with an elicited tachycardia. Electrophysiologic studies can be used to exclude atrial tachycardias originating close to the sinus node. Further, beta-blockers and calcium channel blockers can be used to treat IST, although typically with imperfect results. Radiofrequency (RF) modification of the sinus node may be considered if drug therapy fails, but currently this treatment has limited efficacy and high recurrence rates.
28.5.3 Paroxysmal Supraventricular Tachycardias
PSVTs are a group of SVTs associated with sudden onset and termination. They are usually recurrent and often occur in otherwise seemingly healthy individuals. As a group, PSVTs are among the arrhythmias most amenable to catheter ablation.
28.5.3.1 Sinus Nodal Reentry Tachycardias
Sinus nodal reentry tachycardias are relatively rare (i.e., accounting for approximately 3 % of all PSVTs), and tend to occur mainly in older individuals with other manifestations of sinus node disease. The average rate of sinus node reentry tachycardia is 130–140 bpm, yet these rates can be quite labile, suggesting that autonomic influences may be at play. By definition, the P-wave morphology is identical or very similar to the sinus P-wave. Vagal maneuvers can slow or terminate these tachycardias, since they reenter within a region of the sinus node that is heavily influenced by vagal (parasympathetic) nerve endings. Further, a sinus nodal reentry tachycardia should be suspected in “anxiety-related sinus tachycardia.” Beta-blockers and calcium channel blockers (e.g., verapamil, diltiazem), as well as ablation, all remain as viable treatment options.
28.5.3.2 Atrial Tachycardias (ATs)
Atrial tachycardias (ATs) refer to those tachyarrhythmias that arise in atrial tissues and which are due to abnormal automaticity or reentry. They are classified separately from sinus node reentry or AV nodal reentry because, although these latter tachyarrhythmias also arise within or utilize the atria, the sinus node and AV node are critical components of their respective re-entrant tachycardias. A typical AT has an atrial rate of 150–200 bpm with a P-wave morphology that is usually different from that of sinus P-wave; ATs account for 5–10 % of all PSVTs.
Since ATs arise within and are sustained by atrial tissues alone, AV block may develop without interrupting the tachycardia. The later is, in fact, one of the most valuable ways to distinguish ATs from other SVTs, in that they are AVN-dependent such as AV nodal reentry. ATs may be due to automaticity or triggered activities as well as reentry. A typical automatic AT has the following features: (1) it cannot be initiated or terminated by atrial stimulation; (2) the first P-wave of the tachycardia is the same as the subsequent P-waves of the tachycardia; (3) its rate often accelerates after initiation until it stabilizes (at 100–175 bpm), the so-called warm–up phenomenon; (4) a premature atrial stimulation can reset automatic AT with a full or incomplete compensatory pause, usually accompanied by a constant return cycle; and/or (5) overdrive suppression is a hallmark of automaticity. Currently, both reentrant and automatic ATs are treated with ablation as a first line therapy.
28.5.3.3 AV Nodal Reentry Tachycardias (AVNRTs)
An AV nodal reentry tachycardia (AVNRT) is the most common of PSVTs (representing 50–65 % of all such arrhythmias) and usually presents as a narrow QRS complex with regular rates between 120 and 250 bpm. In the absence of ventricular preexcitation (i.e., Wolff–Parkinson–White or WPW syndrome and related disorders), AVNRTs and AV reentry tachycardias (see below) typically utilize a concealed bypass tract in >90 % of all PSVTs cases.
A schematic of a typical AV nodal reentry circuit is shown in Fig. 28.2. The AV node has two functional pathways with different conduction velocities and refractory periods. In normal sinus rhythm, conduction is usually over the faster of the two pathways. During the tachycardia, retrograde P-waves may not be apparent because their signals are buried in the QRS complexes or they appear as subtle distortions at the terminal parts of the QRS complex. In general, AVNRTs can be reproducibly initiated and terminated by appropriately timed atrial extra stimuli; this is routinely done as part of the diagnostic electrophysiologic study (EPS) in patients. Spontaneous PACs that initiate an AVNRT are usually associated with a significantly prolonged PR interval due to prolonged conduction within the AV node. In intracardiac recordings during EPS, this is demonstrated by a prolonged “AH” interval, the conduction time between the local atrial tissue adjacent to the AV node and the activation of the His bundle. This PR (or AH) prolongation represents refractoriness of the faster AV nodal pathway with resultant conduction on the so-called slow AV nodal pathway. If the conduction delay in the slow AV nodal pathway (Fig. 28.2) is sufficient to allow recovery of the fast pathway and permit the fast pathway to conduct retrogradely back toward the atrium, the reentry circuit is completed and tachycardia may ensue. Commonly, a critical balance between conduction delay and recovery of refractoriness in these two pathways is required to sustain the tachycardia. Simultaneous antegrade conduction to the ventricles and retrograde conduction to the atria often lead to the “retrograde” P-waves being hidden within the QRS complex, as noted earlier. Furthermore, as a consequence of this physiology, in typical “slow-fast” AVNRT the RP interval is short (i.e., <80–100 ms) in AVNRT (Fig. 28.3). In contrast, in AVRT (i.e., a PSVT using an accessory AV connection such as in WPW syndrome), the ventricles and atria are activated sequentially and the RP interval is expected to be longer than 80 ms (Fig. 28.4).
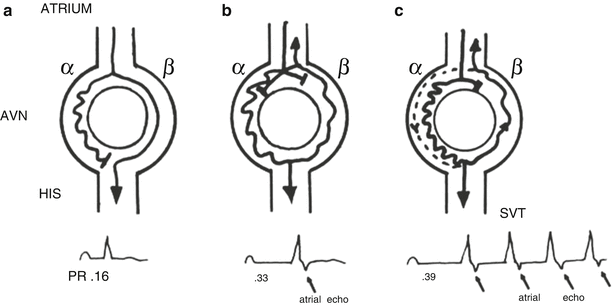
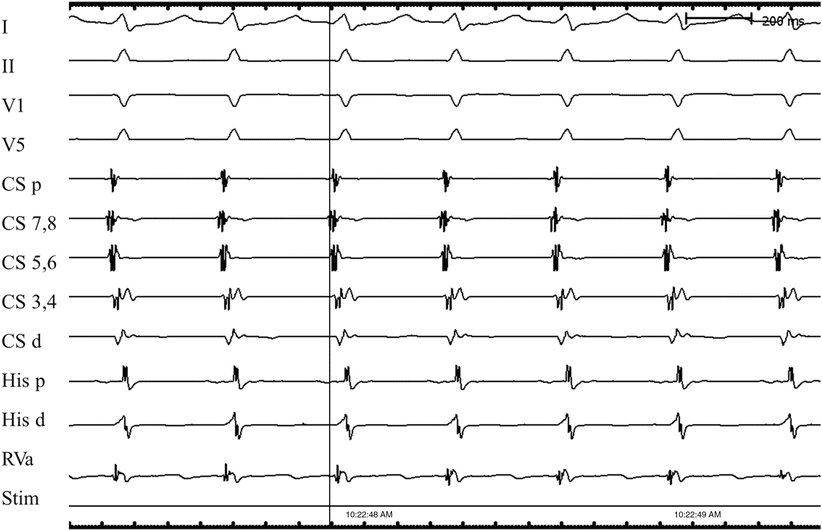
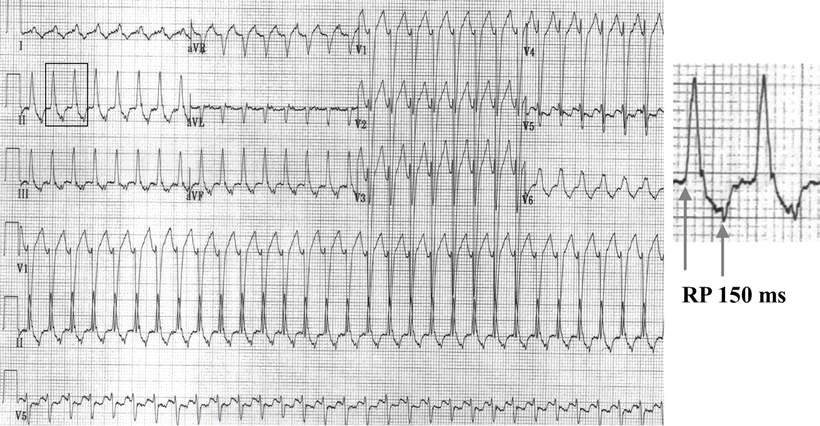

Fig. 28.2
Schema of the typical atrioventricular nodal reentry tachycardia (AVNRT). The atrioventricular node (AVN) has a slow pathway with short refractoriness and a fast pathway with long refractoriness. (a) During sinus rhythm, the impulse conducts to the ventricles through the fast pathway, yielding a normal PR interval. The impulse simultaneously goes down the slow pathway, but cannot conduct to the His bundle antegradely or retrogradely to the fast pathway since they are rendered refractory by the prior beat. (b) An atrial premature complex reaches the effective refractory period of the fast pathway and is blocked in the fast pathway. This atrial premature complex is able to conduct slowly down to the slow pathway, yielding a prolonged PR interval. The delay in conduction over the slow pathway allows enough time for the fast pathway to recover and enables the impulse conducted from the slow pathway to continue over the fast pathway retrogradely to the atria, producing an atrial echo beat. At the same time, the returned impulse tries to conduct down over the slow pathway and fails due to unrecovered refractoriness of the slow pathway. (c) A sufficient early atrial premature complex occurs, producing a similar echo beat as in (b). However, the returned impulse is able to conduct down over the slow pathway, repeatedly producing another ventricular beat and atrial echo, i.e., supraventricular tachycardia (SVT). Reproduced from reference [4] with permission. ©1993, Clinical cardiac electrophysiology: techniques and interpretations, 2nd edition. Reproduced with permission by Wolters Kluwer

Fig. 28.3
Typical atrioventricular (AV) nodal reentry tachycardia. Since the tachycardia circuit is within the AV node, and it conducts simultaneously antegrade to the ventricles and retrograde to the atria, the time difference between ventricular and atrial activation is relatively short. The retrograde P-wave is often buried in QRS complex (RP interval is 0 ms), as shown in this case. I surface ECG lead I, II surface ECG lead II, V1 surface ECG lead V1, V5 surface ECG lead V5, CS coronary sinus, d distal, His His bundle, p proximal, RVa right ventricular apex, stim stimulation

Fig. 28.4
Typical atrioventricular reentry tachycardia. Note that the retrograde P-wave is 150 ms after the onset of QRS complex (RP interval is 150 ms)
Acute treatment to terminate AVNRT includes vagal maneuvers (e.g., carotid sinus massage, Valsalva maneuver), adenosine injections, administration of verapamil or diltiazem or beta-blocker, or electrical cardioversion. The majority of these interventions are designed to interrupt AV nodal conduction transiently, thereby “breaking” the fragile reentry circuit. Drugs used for long-term prevention of AVNRT recurrence include digitalis (not recommended currently due to low efficacy), beta-blockers, calcium channel blockers, and Class Ia and Ic antiarrhythmic drugs. However, catheter ablation (principally of the “slow” pathway region) is currently the first line therapy, as it has a success rate of more than 95 % with relatively low risk.
28.5.3.4 AV Reentry Tachycardias (AVRTs) using an Accessory Pathway
AV reentry tachycardia (AVRT) which uses an accessory pathway is another common form of PSVT. In these patients, accessory conduction tissue remaining from embryonic development of the heart can create the substrate for reentry PSVT. The most common form of an accessory pathway is one connecting the atria to the ventricles (i.e., an accessory AV connection). Such a connection is typically composed of working muscle tissue that is so small that it is usually undetectable during open-heart surgery. When these connections conduct in the antegrade direction (i.e., from atrium to ventricle), they modify the first part of the QRS configuration, usually by virtue of earlier than expected activation of the connected part of the ventricular muscle (i.e., preexcitation). The classic case is the “delta” wave observed at the onset of the QRS in WPW syndrome (see below).
In many patients, accessory connections only conduct in the retrograde direction (from ventricle to atrium) and are known as concealed accessory connections. In these cases, there is no apparent ECG footprint, since ventricular preexcitation does not occur. Nevertheless, since retrograde conduction can occur, a reentry tachycardia is possible. This form of accessory pathway most often occurs on the left side of the heart, and accounts for approximately 30 % of all PSVTs. In general, the electrical impulses in this type of PSVT circulate in an antegrade direction through the AV node and retrograde through the concealed accessory pathway (Fig. 28.5). In other words, both the atria and ventricles are parts of the reentry circuit. Since atrial activation always follows ventricular activation, the P-wave usually occurs after the QRS complex and the RP interval is relatively long (RP interval >80 ms, but usually in the range of 120 ms).
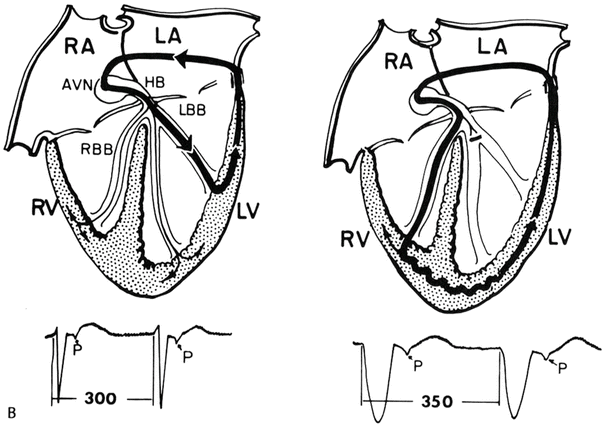

Fig. 28.5
Atrioventricular reentry tachycardia using a left-sided concealed accessory pathway (left). Left bundle branch block prolongs the tachycardia cycle length by 50 ms due to the conduction delay of the tachycardia circuit in the left ventricle (right). Reproduced from reference [4] with permission. AVN atrioventricular node, HB His bundle, LA left atrium, LBB left bundle branch, LV left ventricle, RA right atrium, RBB right bundle branch, RV right ventricle. ©1993, Clinical cardiac electrophysiology: techniques and interpretations, 2nd edition. Reproduced with permission by Wolters Kluwer
An AVRT can be initiated and terminated by either an atrial or ventricular extra stimuli. Even when the His bundle is refractory, a PVC may be able to reset the atria by virtue of being transmitted to the atria through the accessory pathway(s). In contrast to the concentric atrial activation sequence in AVNRT, the atrial activation sequences in AVRT are eccentric when the accessory pathways are located at a distance from the AV node. On occasion, however, the connection may be close to the AV node, making the distinction between the two arrhythmias clinically more challenging.
Medical treatment of an AVRT is similar to that of AVNRT. However, transcatheter ablation is highly effective (>95 %) for eliminating accessory AV connections and has become the preferred approach, especially in younger individuals.
28.5.3.5 Wolff–Parkinson–White (WPW) Syndrome and Related Preexcitation Syndromes
When there are one or more accessory AV pathways or connections that conduct in the antegrade direction, the ventricles may become overtly preexcited to a varying degree, as discussed earlier. The term WPW syndrome is applied when palpitations/tachyarrhythmias occur in patients with overt preexcitation during sinus rhythm. Tachycardias associated with WPW syndrome include: (1) orthodromic reciprocating or reentry tachycardia, in which the conduction path is antegrade through the normal AV conduction system and retrograde through the AV accessory pathways; (2) antidromic reciprocating or reentry tachycardia, in which the conduction path is antegrade through the AV accessory pathways and retrograde through the normal AV conduction system or another accessory connection; and (3) separate pre–excited tachycardia (AFib or flutter, atrial tachycardia, or AVNRT), in which the activation of the ventricles occurs antegrade through both the normal AV conduction system and AV accessory pathways. In these last cases, the accessory pathways typically do not play a critical role in sustaining the tachycardia and thus are not part of the circuit. The ECG features of a typical AV connection in WPW syndrome (Fig. 28.6) are: (1) shortened PR intervals, <120 ms during sinus rhythm; (2) widened QRS durations; and (3) presence of delta waves (a slurred, slowly rising onset of the QRS). Note that the terminal QRS portion is usually normal in appearance, yet sometimes it is associated with secondary ST-T changes.
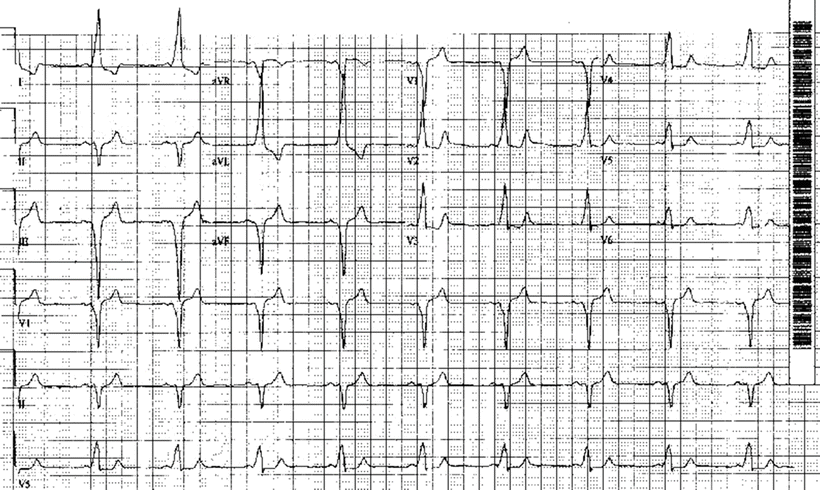

Fig. 28.6
Wolff–Parkinson–White syndrome using a right posterior septal accessory pathway. See text for discussion
In addition to typical AV accessory pathways, where the accessory connection is between the atrium and the ventricle, other variants may exist, including: (1) atriofascicular (also called Mahaim fibers), in which there is a connection between the atrium and one of the bundles of the conduction system; (2) fasciculoventricular, where there is a connection between one of the bundles and the ventricle outside the normal Purkinje fibers; and (3) the very rare nodofascicular and nodoventricular fibers. Fasciculoventricular fibers typically cause subtle pre-excitation but do not cause tachycardia.
The majority of the atriofascicular connections are long right-sided pathways, commonly at the lateral and anterolateral tricuspid annulus and connecting the right atrium to the right bundle branch or the right ventricular free wall. These fibers almost represent a duplication of the AV nodal pathway and are typically capable of only decremental antegrade conduction [5], therefore, they can only cause preexcited tachycardia. In such a patient, often preexcitation is not initially apparent, but can be exposed via right atrial stimulation. Importantly, the associated ECG tends to exhibit a left bundle branch block type of morphology, with a leftward frontal axis similar to that associated with right ventricular apical pacing.
An incessant form of SVT has been described that is usually associated with a slow conducting posteroseptal atrioventricular accessory pathway as its retrograde limb (the so-called permanent junctional reciprocating tachycardia). This tachycardia tends to be more often observed in children than in adults. Importantly, its sustained nature can result in deterioration of left ventricular function over time. Hence, an early diagnosis is crucial to prevent subsequent heart failure, and ablation therapy in such patients is currently the treatment of choice.
It is important to note that the mere presence of an accessory pathway does not necessarily mean that it is involved in the underlying mechanism of presented tachycardia in a given patient. Not infrequently, these pathways behave as innocent bystanders, yet during the elicitation of AFib they create additional stress on the heart by permitting conduction of very rapid rates to the ventricles. Therefore, one needs to consider that multiple tachycardia mechanisms may reside in a patient simultaneously. For example, AV nodal echoes or AVNRT may be present in 15–20 % of patients after successful ablation of accessory pathways; moreover, up to 10 % of patients with AVRT have multiple accessory connections.
It is estimated that 10–35 % of patients with preexcitation will experience an AFib episode at some point in time. In fact, AFib and atrial flutter may be the presenting arrhythmia in up to 20 % of patients with accessory AV pathways. In patients with WPW syndrome, atrial tachyarrhythmias, such as AFib, may conduct to the ventricles via the accessory pathway and produce an extremely rapid ventricular response that then may degenerate into ventricular fibrillation. Importantly, a grossly irregular RR interval with widened QRS complexes and extremely rapid ventricular rates should immediately suggest the presence of antegrade accessory AV pathway conduction that is associated with AFib (Fig. 28.7). It is considered that patients with preexcited AFib are at increased risk of developing ventricular fibrillation if the shortest preexcited RR interval during AFib is less than 250 ms. Intravenous procainamide is the acute treatment of choice for hemodynamically stable preexcited AFib (but it must be administered slowly to avoid hypotension). Note, intravenous AV nodal blocking agents, such as digoxin and verapamil, are contraindicated in these patients because they may paradoxically enhance antegrade AV conduction via the accessory pathway, resulting in hypotension or even cardiac arrest. Patients who are hemodynamically unstable may benefit from immediate cardioversion. Among patients with WPW, many episodes of AFib may result from degeneration of orthodromic AV tachycardia (tachycardia–induced tachycardia). In general, a successful ablation of the accessory AV pathway often eliminates AFib in these patients. Furthermore, RF ablation of the accessory pathway is the treatment of choice for symptomatic patients with WPW syndrome. It is safe and effective in more than 95 % of patients. For more details on the technological methods for ablation, see Chap. 29.
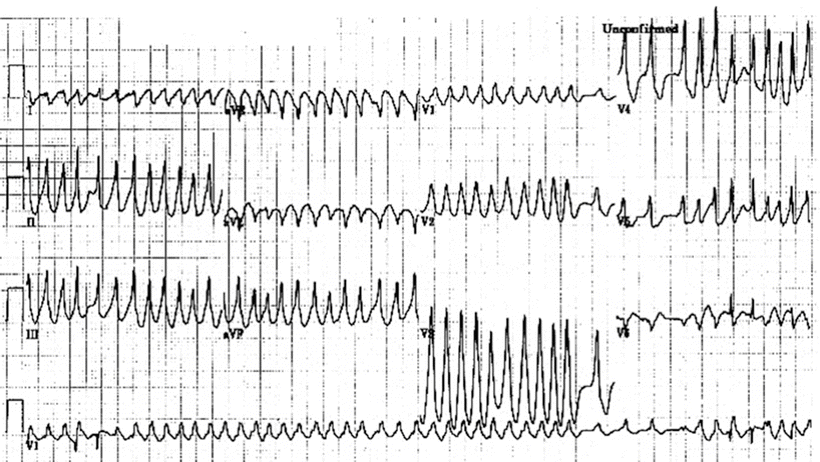

Fig. 28.7
Preexcited atrial fibrillation. See text for discussion
28.5.4 Atrial Flutter and Fibrillation
28.5.4.1 Atrial Flutter
In general, atrial flutter is characterized by an atrial electrical rate between 250 and 350 bpm usually accompanied by 2:1 AV conduction, and thus resulting in ventricular rates of approximately 150 bpm. Classical flutter waves (F–waves) are regular sawtooth-like deflections within the ECG, most prominent in the inferior leads and sometimes V1 (Fig. 28.8). A typical F-wave may appear to be similar to one generated by other atrial tachycardias with rates between 200 and 300 bpm. Yet, the flutter rates can be as low as 200 bpm in some patients, particularly in the presence of antiarrhythmic drug therapy. Although antiarrhythmic drugs may be useful to prevent recurrence of atrial flutter, they are not very effective for conversion back to sinus rhythm. To date, DC electrical cardioversion (i.e., utilizing 50–100 joules) has been the most effective means for termination of atrial flutter.
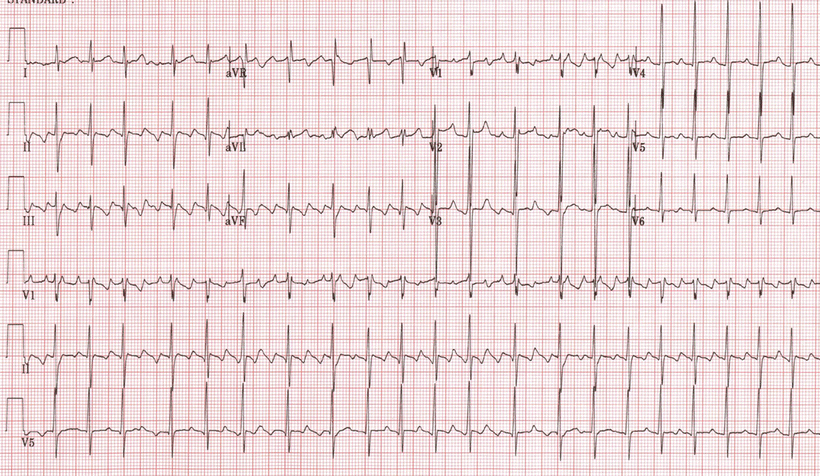

Fig. 28.8
Atrial flutter. See text for discussion
Typically, atrial flutter is a macroreentrant rhythm confined to the right atrium. In most cases, the tachycardia circulates around the tricuspid annulus but passes through a relatively narrow isthmus of tissue between the inferior vena cava and the tricuspid valve annulus (cavotricuspid isthmus). Therefore, creating conduction block with ablation within this isthmus is a highly effective means for terminating the tachycardia and also avoiding recurrence. Therefore, ablation in such cases remains the treatment of choice. Although systemic embolization is less common in atrial flutter than in AFib, anticoagulation for patient with atrial flutter should also follow the recommended guidelines for the management of AFib (see below) [6, 7].
28.5.4.2 Atrial Fibrillation
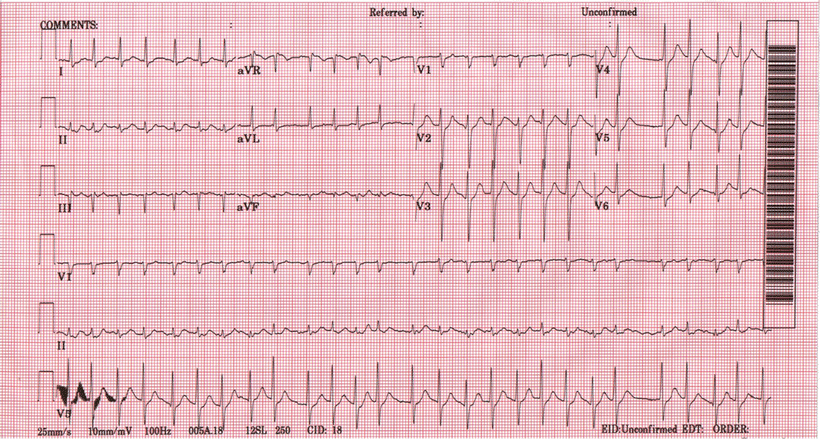
In general, AFib is an uncoordinated atrial tachyarrhythmia characterized by ECG recording as an absence of distinct P-waves before each QRS complex, the presence of rapid atrial oscillations, and variable RR intervals (Fig. 28.9). Paroxysmal AFib is arbitrarily defined as AFib that terminates spontaneously or with intervention within 7 days of onset. Persistent AFib is defined as AFib lasting longer than 7 days. If a number of attempts of termination by cardioversion have failed, or if the patient and physician have decided to cease further attempts to restore and/or maintain sinus rhythm, AFib is regarded as permanent. When no prior history of AFib is available, the term recent or new onset is often employed.
 < div class='tao-gold-member'>
< div class='tao-gold-member'>





Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


