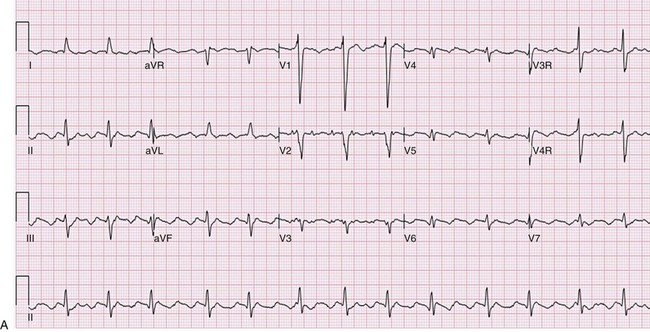
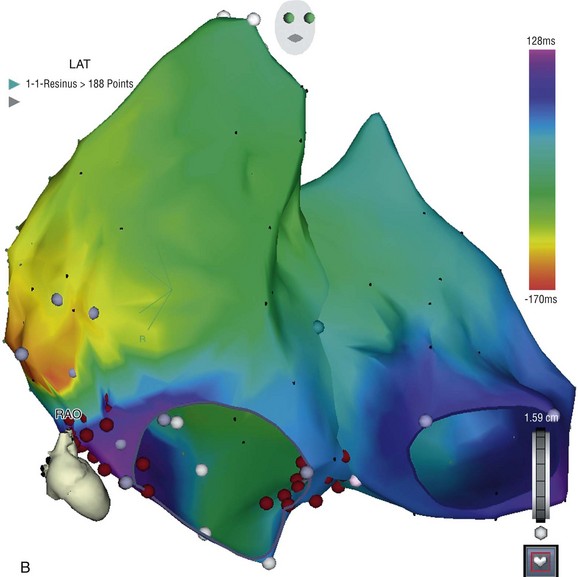
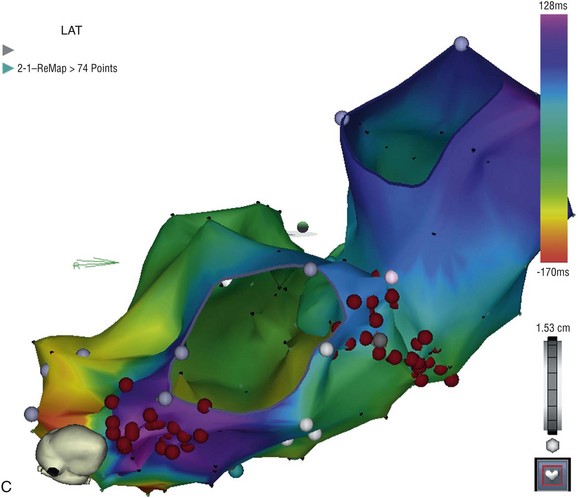
127 The indications for catheter ablation in adults and children have become gradually less restrictive over the past two decades, coincident with published data demonstrating safety and efficacy of the procedure.1–3 In the past, the recommendations were to begin treatment with a trial of medications; however, catheter ablation is now considered a reasonable first-line approach for the treatment of symptomatic arrhythmias in adults and older children.4–6 More urgent indications for ablation include the presence of ventricular dysfunction, a history of syncope, or other concerning symptoms. There may be contraindications to β-adrenergic blockers or other antiarrhythmic medications, or confounding medical conditions (e.g., asthma, depression), particularly in the concomitant presence of structural CHD. Although some practitioners choose to trial first-line medications such as β-blockers, calcium-channel blockers, or digoxin, these have relatively low efficacy compared with more potent antiarrhythmic agents, such as sodium-channel blockers (e.g., flecainide) or potassium channel and multichannel blocking agents (e.g., sotalol, amiodarone). There are risks and benefits of long-term medical therapy, and these need to be weighed against the risks and benefits of catheter ablation. The choice of catheter ablation for children older than 3 to 5 years of age and weighing at least 15 to 20 kg is certainly reasonable, with high efficacy and relatively low rates of serious complications in experienced hands.7–11 It is also reasonable to proceed with catheter ablation in patients before planned congenital heart surgery to reduce the potential complications associated with perioperative arrhythmias and to be able to access sites that might be isolated by the surgical repair. For example, patients with Ebstein anomaly, Wolff-Parkinson-White syndrome, or supraventricular tachycardia (SVT) might be more optimally managed by preoperative catheter ablation. Currently, there are more adults than children living with CHD, because of marked improvements in early (even fetal) diagnosis, congenital heart surgery, and perioperative management. The indications for catheter ablation in adult and pediatric patients with CHD can vary by arrhythmia substrate and anatomic complexity. Atrial arrhythmias include automatic (ectopic atrial tachycardia), reentrant (atrial flutter and intraatrial reentry tachycardia [IART]) and focal atrial tachycardias, which can also be automatic or reentrant. Atrioventricular reentrant tachycardias can occur in patients with CHD, and there are some distinct associations between congenital structural and electrical abnormalities. Ventricular tachycardias are also seen in patients with CHD, often because of areas around scars and patches or corridors of slow conduction or ischemia. Indications for catheter ablation of atrial, atrioventricular (AV) reciprocating, or ventricular tachycardias in patients with CHD include syncope, other significant symptoms, or hemodynamic compromise. Ablation in patients with CHD is judicious, particularly if they experience antiarrhythmic medication refractoriness or side effects or have contraindications to medical therapy. First-line therapy is also reasonable and probably at clinical equipoise, given the poor efficacy and tolerability of chronic medical therapy. However, long-term outcomes and success rates are typically lower in patients with CHD compared to adults and children with structurally normal hearts.12–15 Standard catheter ablation techniques might be sufficient for many patients with less complex anatomy. However, it is vitally important to apply practices that minimize ionizing radiation exposure to young patients who are particularly susceptible to the damaging effects and potentially have decades in which to develop future malignancy.16,17 These patients also could be exposed to additional sources of radiation during the clinical follow-up and management of their heart disease, including x-rays, nuclear studies, computed tomographic scans, diagnostic and interventional cardiac catheterizations, implantable devices, and repeated catheter ablation procedures. Ablation procedure duration in patients with CHD is often longer than in patients with structurally normal hearts; therefore, general anesthesia is often used. Because of their anatomical and physiological complexity, patients with CHD in particular can benefit from technological advances in mapping and catheter ablation, even if these technologies were not specifically designed with the CHD population in mind. There are several types of three-dimensional nonfluoroscopic electroanatomic or noncontact mapping systems, which allow for the determination of precise localization of arrhythmic substrates and provide a reasonable representation of electrical activation and propagation in each chamber. These mapping systems, described in greater detail elsewhere in this textbook, can create an anatomical shell of each cardiac chamber, and then superimpose the electrical activation patterns within and between these shells. Further anatomical detail can be obtained by merging imaging data acquired from cardiac computed tomography or MRI on to the anatomical three-dimensional shell. These precise anatomical details are critically important for successful electrical mapping in these challenging patients with CHD who often have multiple scars, electrically inert patches, and distinct isthmuses of slow conduction or ischemia (Figure 127-1).18–21 Intracardiac or transesophageal echocardiography can be useful adjunct imaging techniques to further define the anatomical structures and obstacles without additional ionizing radiation. Intracardiac echocardiography is particularly beneficial for identification of landmarks and substrates involved in arrhythmogenesis, such as the crista terminalis, coronary sinus ostium, pulmonary veins, and surgical patches.22 Remote magnetic navigation is discussed elsewhere, but it has been shown in limited numbers to be of utility in mapping complex congenital anatomies and in maneuvering the ablation catheter to difficult locations in patients with CHD.23–25 Currently, this approach is not widely available in pediatric centers, and clinical studies demonstrate equivalent success rates and complications as standard techniques, with perhaps lower fluoroscopy exposure. Figure 127-1 A, Electrocardiogram of young adult with repaired double outlet right ventricle and pulmonary stenosis, more than 10 years postoperatively with an atrial tachycardia. Note that the P waves are not so obvious on every lead, emphasizing the importance of obtaining a 12- to 15-lead electrocardiogram. The tachycardia cycle length is 360 ms with variable (2 : 1 to 3 : 1 AV conduction) and QRS duration of 130 ms because of an intraventricular conduction delay. B, Electroanatomical map of both the right and left atria simultaneously displayed in right anterior oblique angulation to illustrate the atrioventricular valves, and an isthmus of slow conduction between the tricuspid valve and inferior vena cava. A propagation map reveals this to be a clockwise right atrial circuit. C, Electroanatomical map of right and left atria from an inferior view to highlight the target areas for ablation lines in the cavotricuspid isthmus and a second line from coronary sinus to atrial septum. There are several particularly challenging arrhythmias seen in patients with CHD, and interestingly, these can occur decades after surgical repair. For example, IART is occurring with increasing frequency in patients with CHD who are now surviving longer after repair, but are developing arrhythmia sequelae of long-standing hemodynamic pressure and volume abnormalities, combined with anatomical substrates such as scars, patches, and zones of slow conduction (Figure 127-2). IART can be seen years or decades following complex atrial surgery, particularly after the atrial switch procedures (i.e., Mustard, Senning) for dextro-transposition of the great arteries or the Fontan procedure for single ventricle physiology.26,27 Propagation maps illustrate the circuit movement during tachycardia, which can also facilitate comprehension of the planned ablation targeted sites (Figure 127-3). Nonautomatic focal atrial tachycardias can be challenging to differentiate from IART on surface electrocardiogram (ECG), but account for much less frequent arrhythmias in CHD patients (Figure 127-4).28,29 The atrial walls may be significantly thicker in these patients because of chronically elevated intraatrial pressures and volumes, and this has important implications for catheter ablation.30 The lesions may need to be deeper and larger to result in successful ablation, thereby requiring the use of either larger-tip catheters, or the use of irrigated or cooled radiofrequency (RF) ablation to increase the lesion depth.21 The arrhythmogenic circuit can involve tissue on either or both atrioventricular annuli and can require approaches from the systemic circulation (retrograde or transbaffle puncture).31,32 Similarly, ventricular muscle is hypertrophic in certain congenital substrates, such as tetralogy of Fallot, and the use of deeper RF lesions may be necessary to achieve successful outcomes (Figure 127-5). VT in CHD can also be more difficult to induce, limiting mapping density and ablation success.33
Catheter Ablation in Congenital Heart Disease
Indications for Ablation in Congenital Heart Disease
Catheter Ablation Techniques and Technologies in Patients With Congenital Heart Disease
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Thoracic Key
Fastest Thoracic Insight Engine
