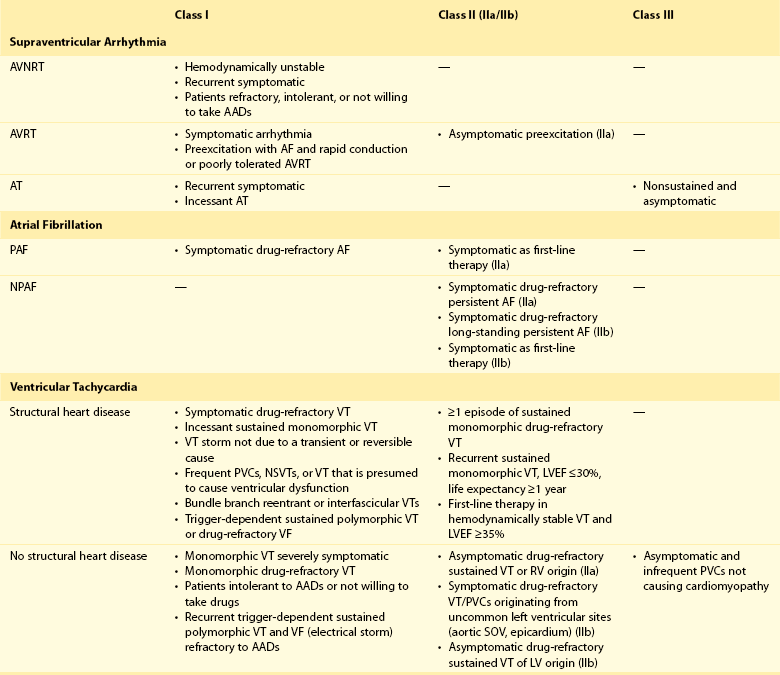
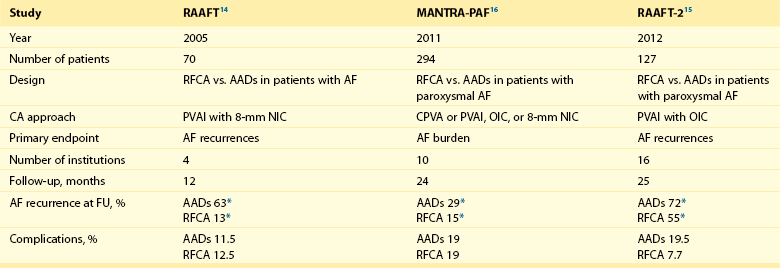
121 Supraventricular arrhythmias encompass a wide group of cardiac rhythm disturbances that are relatively common, typically occur in repetitive bouts, and very rarely are life threatening.1,2 Current guidelines support catheter ablation as a class I indication in patients with symptomatic SVAs who are drug resistant or drug intolerant or do not want to receive long-term drug therapy (Table 121-1).1 Of note, the clinical effectiveness of antiarrhythmic drug therapy in patients with SVAs is typically suboptimal, with patients often experiencing arrhythmia recurrence despite drug treatment; also, the risks associated with long-term therapy with antiarrhythmic drugs are not negligible. Therefore, many clinicians support the appropriateness of considering catheter ablation as first-line therapy for most patients with symptomatic SVAs. Asymptomatic SVAs, on the other hand, very rarely (if ever) constitute an indication for invasive electrophysiological study and catheter ablation, given the overall good prognosis of such arrhythmias. Table 121-1 Guideline Indications for Catheter Ablation of Cardiac Arrhythmias From Blomstrom-Lundqvist C, Scheinman MM, Aliot EM, et al: ACC/AHA/ESC guidelines for the management of patients with supraventricular arrhythmias—executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (writing committee to develop guidelines for the management of patients with supraventricular arrhythmias) developed in collaboration with NASPE-Heart Rhythm Society. J Am Coll Cardiol 42:1493-1531, 2003; Calkins H, Kuck KH, Cappato R, et al: 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: Recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Heart Rhythm 9:632-696, 2012; Aliot EM, Stevenson WG, Almendral-Garrote JM, et al: EHRA/HRS expert consensus on catheter ablation of ventricular arrhythmias. Heart Rhythm 6:886-933, 2009; Natale A, Raviele A, Al-Ahmad A, et al: Venice Chart International Consensus document on ventricular tachycardia/ventricular fibrillation ablation. J Cardiovasc Electrophysiol 21:339-379, 2010. Age and gender exert a significant influence on the prevalence and mechanism of SVA. Age at tachycardia onset is typically higher (average, 30 years) and female gender more prevalent in patients presenting with atrioventricular node reentrant tachycardia (AVNRT) as opposed to those with atrioventricular reentrant tachycardia (AVRT).3 Female gender is also associated with a higher incidence of focal automatic atrial tachycardia (AT), especially during pre-menopause, and with a lower risk of sudden cardiac death in the presence of atrioventricular bypass tracts, essentially caused by the lower incidence of atrial fibrillation (AF) in females with preexcitation syndromes.3 Persistent SVAs, such as focal AT, might also present with signs and symptoms of tachycardia-induced cardiomyopathy. A recent study on 345 patients undergoing radiofrequency catheter ablation of focal AT showed prevalence of tachycardia-mediated cardiomyopathy in 10% of patients, typically in younger males (mean age, 39 ± 22 years vs. 51 ± 17 years in those not developing tachycardiomyopathy; P = .006) with relatively long tachycardia cycle lengths (502 ± 131 ms vs. 402 ± 105 ms; P < .001).4 In this study, catheter ablation restored normal left ventricular function in almost all (97%) patients after a mean follow-up of 3 months. With regard to patients with atrioventricular accessory connections, the clinical presentation may be highly variable, ranging from no symptoms to sudden cardiac arrest. Although some clinicians support an early invasive risk stratification with electrophysiological study in patients with asymptomatic manifest preexcitation because of the possible and unpredictable risk of sudden cardiac death, recent evidence has consistently shown that the actual risk of major cardiac events in patients with asymptomatic preexcitation is extraordinarily low, which calls into question the appropriateness of routine invasive management in these patients.2,5 On the other hand, as is the case for other SVAs, the combination of clinical tachycardia and evidence of preexcitation on the electrocardiogram constitutes a class I indication for invasive electrophysiological evaluation.1 When the ablation procedure is planned for patients with accessory pathways, several clinical and electrocardiographic characteristics are useful for predicting the ablation approach, as well as the procedural success and possible complications. From a clinical standpoint, it is important to emphasize that preexcitation syndromes can be associated with underlying structural heart disease, which might pose unique challenges during catheter ablation. Ebstein’s anomaly of the tricuspid valve is the most common form of structural heart disease associated with accessory pathways (up to 10% to 30% of cases), which are typically right-sided and often multiple.6 In these patients, catheter ablation of accessory pathways has been reported to have a slightly lower success rate, likely as the result of impaired catheter stability in the presence of an anatomically abnormal right atrioventricular groove.6 Univentricular heart syndromes, atrial septal defects, and congenitally corrected transposition of the great vessels are among other congenital heart diseases associated with the presence of accessory pathways. Rarely, accessory atrioventricular connections could be iatrogenically provoked through surgical anastomosis of atrial tissue to the underlying ventricular myocardium when the Bjork variant of the Fontan operation is performed.7 In these cases, an endocardial-only approach might be insufficient, given the possibility of epicardial connections at the level of the surgical anastomosis. A careful collection of family history of Wolff-Parkinson-White syndrome is important for disclosing rare forms of familial syndromes often associated with glycogen storage cardiomyopathy in the setting of a mutation in the PRKAG2 gene.8 Clinically, such patients typically display evidence of accessory atrioventricular connections (often fasciculoventricular pathways) and signs of left ventricular hypertrophy8 and are characterized by a high incidence of other cardiac rhythm disturbances such as sick sinus syndrome and complete atrioventricular block. For planning of the ablation procedure in patients with an accessory pathway, proper recognition of pathways with high risk of iatrogenic atrioventricular block during ablation (i.e., anteroseptal or midseptal pathways) or of those requiring special approaches such as epicardial access for mapping and ablation is of utmost importance. Anteroseptal and midseptal pathways are typically characterized by a negative delta wave in lead V1 with transition before lead V3 and a frankly positive polarity in the inferior leads (i.e., DII, DIII, and aVF).9,10 Some of these pathways are classified as para-Hisian; according to their initial description, a clear His bundle deflection (usually >0.1 mV) should be recorded on the ablation catheter in these cases, and the tip of the ablation catheter should be overlapping with the tip of a diagnostic His bundle catheter on any fluoroscopic view.11 Therefore, by definition, para-Hisian pathways cannot be diagnosed on the basis of electrocardiographic criteria only. Nonetheless, anteroseptal or midseptal pathways are usually easily detected by means of electrocardiographic criteria; this is important for correctly informing patients about the estimated success and possible complications of the procedure and for planning in advance ablation with alternative sources of energy, such as cryoenergy (see Chapter 121). Few clinical and electrocardiographic criteria have been proposed for selection of patients who are likely to require epicardial access for mapping and ablation of accessory pathways. Usually, these patients present after multiple failed endocardial procedures or have some peculiar clinical clues suggestive of epicardial atrioventricular connections, such as surgical anastomosis after the Fontan operation, as was previously discussed. With regard to electrocardiographic criteria, some authors have suggested that a steeply negative delta wave in lead DII in the setting of a posteroseptal accessory pathway predicts the need for epicardial mapping and ablation,9,11 although this criterion has not been adequately validated in large cohorts of patients. Focal ATs might also originate from the atrial subepicardial tissue, although no clinical criteria have been demonstrated that can predict the need for epicardial access in these patients. The 2011 edition of the American College of Cardiology (ACC)/American Heart Association (AHA)/Heart Rhythm Society (HRS) guidelines for the management of AF gives a class I indication with the highest level of evidence to catheter ablation in patients with symptomatic paroxysmal AF who have failed treatment with an antiarrhythmic drug (see Table 121-1).12 Such a position has been endorsed also in the latest Heart Rhythm Society (HRS)/European Heart Rhythm Association (EHRA)/European Cardiac Arrhythmia Society (ECAS) expert consensus statement on catheter and surgical ablation of AF.13 In the near future, the recommendation will likely be extended to patients who have not yet failed any antiarrhythmic drug (i.e., first-line therapy), given the encouraging results of three recent randomized trials comparing catheter ablation with antiarrhythmic drugs for first-line therapy of paroxysmal AF (Table 121-2).14–16 Table 121-2 Baseline Characteristics and Main Outcomes of Randomized Clinical Trials Evaluating Catheter Ablation as First-Line Therapy for Paroxysmal Atrial Fibrillation Updated guidelines have also introduced new recommendations for catheter ablation in patients with nonparoxysmal AF (see Table 121-1). Evidence suggests that catheter ablation is successful in patients not yet included in guideline recommendations, such as those with previous cardiac surgery or valvular heart disease17 and subgroups such as elderly and women.3,18 In brief, catheter ablation should be offered to virtually all patients with symptomatic drug-refractory AF. As will be highlighted later in the chapter, the type of AF, namely, paroxysmal versus nonparoxysmal, is the major clinical determinant of the procedural approach. On the other hand, some clinical features might predict the need for more extensive ablation beyond pulmonary vein antrum isolation (PVAI) because of the presence of non–pulmonary vein (PV) triggers. Among these, female patients,3 elderly patients,18 patients with mechanical cardiac valves17 or corrected atrial septal defects,19 and patients with metabolic syndrome20 or obstructive sleep apnea21 constitute subgroups in which a high prevalence of non-PV triggers has been reported. According to the latest EHRA/HRS expert consensus document, catheter ablation of ventricular arrhythmias (VAs) is recommended in patients with symptomatic sustained monomorphic ventricular tachycardia (VT) refractory to antiarrhythmic drug therapy, in those with bundle branch reentrant or fascicular reentrant VT, and in patients with drug-refractory VT storm not due to a transient cause (see Table 121-1).80 Data also support the benefit of early adoption of catheter ablation in patients with post-infarct VT, although no evidence indicates that such a strategy improves long-term freedom from recurrent arrhythmia as compared with conventional ablation of drug-refractory VT (Figure 121-1).22 Figure 121-1 Individual and Pooled VT Recurrence Rates After Catheter Ablation of Post-Infarct VT From a clinical perspective, a correct diagnosis of the substrate underlying VAs is crucial, as it bears relevant prognostic information. Unfortunately, current noninvasive techniques including cardiac magnetic resonance can miss subtle cardiomyopathic substrates underlying VAs, and more sophisticated invasive tests such as electroanatomical mapping with biopsy might be necessary when clinical suspicion of underlying structural heart disease is high.23,24 The substrate underlying VAs has been demonstrated to be a powerful predictor of the most appropriate procedural approach. Patients with nonischemic cardiomyopathies, including arrhythmogenic right ventricular cardiomyopathy (ARVC),25 hypertrophic cardiomyopathy,26 and myocarditis,27 have a high rate of epicardial VT substrates. Therefore, it is advisable to program an epicardial access for mapping and ablation from the beginning of the procedure in these patients (Figure 121-2). Such an approach is supported by adequate evidence. For instance, four recent studies compared endo-epicardial ablation with an endocardial-only approach in patients with ARVC.25,28–30 Overall, a total of 160 ARVC patients with drug-refractory VT have been included in such studies, of whom 87 (54%) underwent catheter ablation with an endocardial-only approach and 73 (46%) underwent endo-epicardial mapping and ablation. After a mean follow-up of 32 ± 22 months, VT-free survival was achieved in 24 of 87 (28%) patients treated with an endocardial-only approach, as compared with 52 of 73 (71%) patients treated with an endo-epicardial approach. Similar results have been obtained for other nonischemic substrates, such as idiopathic dilated cardiomyopathy and hypertrophic cardiomyopathy.26,31 Figure 121-2 Preprocedural Planning of the Ablation Approach Based on the Myocardial Substrate Underlying VT On the opposite side, most patients with ischemic substrates may be successfully ablated through an endocardial-only approach, although recent data are increasingly showing the importance of epicardial mapping and ablation in these patients (see Figure 121-2). In our most recent series, we reported evidence of epicardial scar with late and fragmented electrograms in up to one-third of a consecutive series of patients referred to our center for catheter ablation of post-infarct VT.32 Other groups have confirmed the importance of epicardial mapping and ablation in current populations of patients with post-infarct VT, especially in patients with inferior-posterior scars.33 Several electrocardiographic criteria have been proposed to detect the origin of VAs and to plan the most appropriate ablation strategy. A detailed discussion on electrocardiographic criteria to guide VA ablation is provided in other chapters (see Chapter 123). Antiarrhythmic drug therapy should be discontinued for at least 4 half-lives in all patients undergoing catheter ablation of cardiac arrhythmias. In fact, antiarrhythmic drugs significantly interfere with arrhythmia inducibility, might conceal underlying arrhythmogenic areas during substrate mapping, and possibly reduce the effectiveness of catheter ablation procedures. Although adequate drug washout can be achieved in a reasonable time before the procedure (i.e., 4 to 7 days) in most patients treated with conventional antiarrhythmic agents, subjects receiving long-term treatment with amiodarone represent a particular challenge because of the long elimination half-life of the drug (i.e., 58 days on average).34 Recent evidence, however, strongly supports an incremental benefit of preprocedural amiodarone washout.35 In the setting of long-standing persistent AF ablation, preliminary data from our group suggest that amiodarone discontinuation 4 to 6 months before the procedure increases the chance of disclosing—and therefore targeting for ablation—latent non-PV trigger sites, and this translates into better long-term arrhythmia-free survival.35 Whether the benefits of amiodarone discontinuation in patients undergoing catheter ablation of AF might be generalizable to those undergoing VA ablation requires further investigation. No specific preprocedural anticoagulation protocol is required for patients undergoing catheter ablation of SVAs (other than AF) and VAs. As a general rule, patients under long-term treatment with oral anticoagulant agents should discontinue oral anticoagulants during the periprocedural period and bridge with low-molecular-weight heparin for procedures requiring an arterial access or a percutaneous epicardial access. On the other hand, catheter ablation procedures requiring only a transvenous approach can be safely performed under therapeutic anticoagulation.36,37 At our institution, we adopt stringent preprocedural anticoagulation management in patients referred for AF ablation.38 In brief, all patients are started on warfarin as outpatients, at least 2 months before the scheduled procedure, and receive weekly international normalized ratio (INR) monitoring during the 4 to 6 weeks preceding the procedure, with a target INR of 2 to 3. Preprocedural transesophageal echocardiography (TEE) is performed only in patients showing subtherapeutic INR values in the month before the procedure. Patients who demonstrate INR values consistently above 2 in the month before the procedure are directly sent to ablation.38 All patients are type- and cross-matched, and packed red blood cells and fresh frozen plasma are made available for infusion in case of hemorrhagic complications under therapeutic INR. If the preprocedural INR is above 3.5, the anticoagulant effect is partially reversed with one to two units of fresh frozen plasma. In patients with heart failure, however, β-blocker discontinuation has been associated with increased risk for worsening heart failure and mortality.39 In these patients, it is recommended to continue β-blocker therapy in the periprocedural period. Similarly, patients with risk factors for ischemic heart disease and those with established ischemic heart disease benefit from periprocedural continuation of β-blockers.40 As is highlighted later in the chapter, in these patients, isoproterenol testing should be avoided. Other drug treatments appropriate for the underlying concomitant cardiac condition, such as antihypertensive medications, heart failure therapies (e.g., renin-angiotensin system blockers), and lipid-lowering drugs, should be maintained during the periprocedural period.41 In recent years, general anesthesia has gained increasing interest, given the increasing numbers of complex and long procedures, such as catheter ablation of AF or VA. The main advantages of general anesthesia are the almost complete abolition of pain and patient awareness, the abolition of patient movement for prolonged periods, and complete control of ventilation. On the other hand, general anesthesia can induce physiological fluctuations, such as drops in blood pressure and/or reflex tachycardia, which might require active intervention. Thus far, only one randomized trial of 257 consecutive patients has formally evaluated the outcomes of conscious sedation as compared with general anesthesia in patients undergoing catheter ablation of AF.42 General anesthesia was initiated with propofol (2 mg/kg) and fentanyl (1 to 2 µg/kg), followed by a neuromuscular blocking agent (usually rocuronium 0.6 to 1 mg/kg) and by endotracheal intubation with intermittent positive-pressure ventilation. Conscious sedation was attained with fentanyl or midazolam. After a mean follow-up of 17 ± 8 months, 88 (69%) patients assigned to conscious sedation were free of atrial arrhythmias when off antiarrhythmic drugs, as compared with 114 (88%) patients randomly assigned to general anesthesia (P < .001) (Figure 121-3). All patients with recurrence underwent a second procedure. Notably, at the repeat procedure, 42% of PVs in the conscious sedation arm had recovered PV conduction, compared with 19% in the general anesthesia group (P = .003).42 Therefore, current evidence supports an incremental benefit of general anesthesia as compared with conscious sedation in patients undergoing catheter ablation of AF. Other strategies to achieve better catheter stability, such as high-frequency jet ventilation,43 have been proposed as a valuable alternative to conscious sedation. On the other side, different complications have been specifically associated with high-frequency jet ventilation, including problems with humidification and warming, high gas flow rates, and rapid increases in lung volume that might cause lung injury through the generation of shear forces at the interface of compliant and atelectatic airways.44 Furthermore, an extensive learning curve is needed for one to become proficient at using this technique safely, and few anesthesia providers have sufficient experience for the technique to be considered the standard of care. Figure 121-3 Impact of general anesthesia on catheter ablation success rate at a mean follow-up of 17 ± 8 months and on rate of pulmonary vein (PV) reconnection at repeat procedure. (Modified from Di Biase L, Conti S, Mohanty P, et al: General anesthesia reduces the prevalence of pulmonary vein reconnection during repeat ablation when compared with conscious sedation: Results from a randomized study. Heart Rhythm 8:368-372, 2011.) The modified Seldinger technique is the preferred method for obtaining vascular access during electrophysiological procedures. Typically, femoral venous accesses in the right and left groin are used to position most diagnostic catheters, the intracardiac echocardiography (ICE) catheter, and the ablation catheter when procedures are performed through a transvenous approach. The major contraindication to femoral vein catheterization is the presence of acute or recurrent ileofemoral deep vein thrombosis. In some patients, such as those with history of recurrent femoral vein catheterization or those with history of radiation therapy, the femoral vein might be partially or totally occluded. Sporadic reports of inferior vena cava interruption have been described. In these cases, a superior approach from the right internal jugular vein might be implemented, also for complex ablation procedures (e.g., catheter ablation of AF).45 Although the adoption of special precautions for obtaining venous access, such as the use of ultrasound, appears justifiable in patients undergoing electrophysiological procedures without interruption of therapeutic anticoagulation, the risk of vascular-related bleeding with such an anticoagulation protocol has been consistently demonstrated as smaller compared with warfarin discontinuation and heparin bridging.36 No evidence has been found of an incremental benefit of ultrasound-guided femoral venous access as compared with the standard manual approach. At our institution, over the past 7188 AF ablations performed, ultrasound-assisted femoral venous access was attained in 1079 (15%) cases. No difference in the rate of vascular-related complications was observed between patients who received ultrasound-assisted vascular access and those in whom conventional manual access was performed (3.2% vs. 3.8%; P = .44). With regard to nonfemoral venous access, such as access of the internal jugular vein, published data suggest an incremental benefit of ultrasound guidance as compared with the blind technique.46 In patients undergoing venous access without interruption of therapeutic anticoagulation, the risks associated with a blind puncture of the internal jugular vein represent a major concern, and conventional blind puncture should be discouraged. At our institution, we systematically avoid a blind puncture of the internal jugular vein and perform access under fluoroscopic guidance after advancing a catheter from the femoral vein into the internal jugular vein. This approach can also be helpful in eliminating the risk of pneumothorax by allowing access in the neck at a higher level.
Catheter Ablation
Clinical Aspects
Preprocedural Management
Clinical Indications and Patient Selection
Supraventricular Arrhythmias
Atrial Fibrillation
Ventricular Arrhythmias
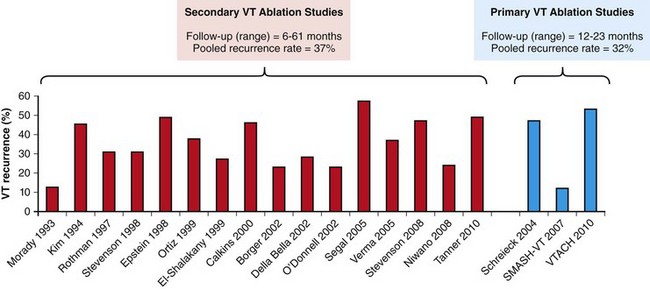
Secondary VT ablation indicates conventional ablation of drug-refractory VT. Primary VT ablation indicates early adoption of catheter ablation, before failure of multiple antiarrhythmic drugs.
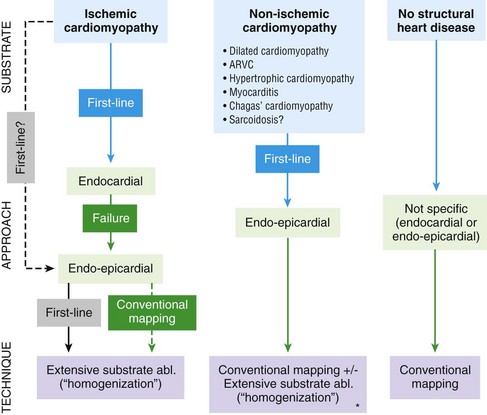
Asterisk indicates that scar is present and the VT is demonstrated to originate from the scar. Conventional mapping indicates conventional entrainment/activation mapping and pace mapping techniques. Extensive substrate ablation (Abl.) indicates ablation of all abnormal electrograms within the scar (fragmented and late electrograms) (see text for details).
Preprocedural Drug Therapy
Antiarrhythmic Drug Therapy
Preprocedural Anticoagulation
Other Drugs
Intraprocedural Management
Sedation and Anesthesia
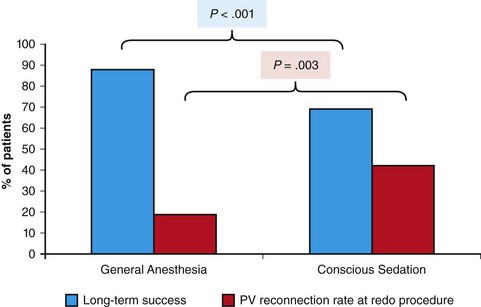
Vascular Accesses, Catheter Manipulation, and Intraprocedural Imaging Techniques
Venous Access
Catheter Ablation: Clinical Aspects



