Chapter 62 Carotid Sinus Stimulation
Background, Technique, and Future Directions
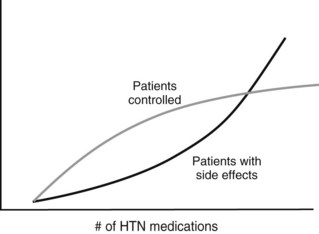
Hypertension affects more than 65 million people in the United States and is one of the most important risk factors for stroke, heart attack, vascular disease, and death. Cardiovascular risk is estimated to double with each 20 mm Hg increment above 115 mm Hg of systolic pressure.1,2 Unfortunately, and despite intensive public health efforts and generally effective pharmacologic therapy, control remains poor,3 in part because adding more antihypertensive medications after a certain point causes side effects to increase while efficacy plateaus (Figure 62-1). In addition, even optimally treated patients who demonstrate perfect compliance can remain significantly hypertensive. A reasonable estimate of those with truly resistant hypertension is 3 to 4 million Americans, and an additional 25 million escape treatment altogether (Figure 62-2).
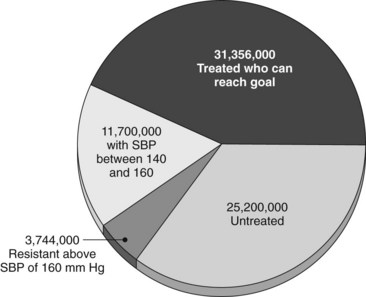
FIGURE 62-2 Proportions and numbers of Americans in various stages and categories of treatment for hypertension.
(Courtesy CVRx, Minneapolis, Minn. Derived from Cushman WC, Ford CE, Cutler JA, et al: Success and predictors of blood pressure control in diverse North American settings: the Antihypertensive and Lipid-Lowering treatment to prevent Heart Attack Trial (ALLHAT), J Clin Hypertension 4:393-404, 2002; and Hajjar I, Kotchen TA: Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988-2000, J Am Med Assoc 290:199-206, 2003.)
One of the major physiologic systems affecting blood pressure (BP) is the carotid sinus baroreflex arc. Increased pressure causes the cells at the sinus to stretch, which directly causes increased glossopharyngeal afferent activity and leads to three downstream effects: cardiac inhibition (decreased stroke volume and heart rate), vascular smooth muscle inhibition (vasorelaxation), and increased renal sodium and water excretion (Figure 62-3).4 This system is responsible for the acute hypotension and bradycardia sometimes seen after carotid endarterectomy, because the sinus is stretched after removal of plaque.
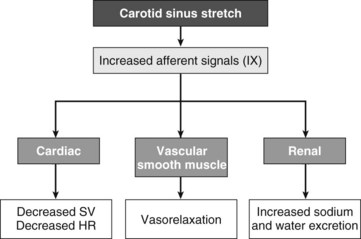
FIGURE 62-3 Schematic diagram of the baroreflex receptor arc.
(Modified from Illig KA, Bisognano J: Carotid stimulation for hypertension, Shelton, Conn, 2010, People’s Medical Publishing House.)
History
The history of device-based therapy for hypertension has been summarized recently.5 As early as 1958, it was reported that electrical stimulation of the carotid sinus nerve in normotensive dogs produced an acute decrease in BP,6 and similar findings were reported in several animal models of hypertension shortly thereafter.7 Following the original animal model report, it was demonstrated that direct electrical stimulation of the carotid sinus in humans (undergoing neck dissection for cancer) had the same results.8
These findings led to more thorough investigation in humans. A single case was reported in 1966 of a 40-year-old man with a BP of 260/165 mm Hg despite four medications. Bilateral stimulation (2.5 V) produced a sustained drop in pressure to 150/90 mm Hg. The device consisted of electrodes at the sinuses connected to a generator placed beneath the pectoralis muscle. The leads were subcutaneously tunneled, and the generator could be turned on or off by placing a magnet over the device, but no further modification could be performed.9 The first series was reported in 1967 Seymour Schwartz; he described a mean BP decrease of 48 mm Hg in eight patients, six of whom reduced their BP medications.10 Further clinical benefit was demonstrated by at least one other group around this time.11 Unfortunately, this treatment came at the wrong time. Most setups at this time required external power sources and communication and were therefore bulky and impractical for long-term use. In addition, pharmacologic therapy dramatically improved in this era, making the problem less acute. As a result, this concept was essentially forgotten for the remainder of the century.
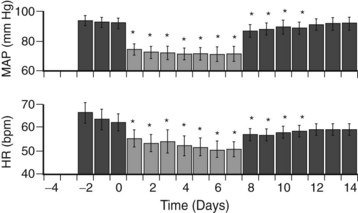
This phenomenon was reevaluated largely through a large body of work by Thomas Lohmeier, a physiologist at the University of Mississippi. In a large series of animal experiments,4,12 he was able to reconfirm that this effect was real and reproducible, was effective in normotensive as well as hypertensive (sodium-loaded and obesity models) canines and, most importantly, was sustainable (Figure 62-4). This work led to the formation of a company (CVRx, Minneapolis, Minn.) whose sole aim was to resurrect this concept using twenty-first–century technology. To date, five discrete trials have been completed or are underway to investigate this effect.
BaroReflex Activating System Study
The BaroReflex Activating System Study (BRASS) was a proof-of-concept trial performed in Switzerland in 2003. Eleven patients undergoing carotid endarterectomy were tested by direct carotid sinus stimulation, and a mean systolic BP drop of 18 mm Hg was observed at a maximum of 4.4 V.13 The BRASS showed that this effect was reproducible in relatively normotensive humans and that clinically feasible levels of current delivered unilaterally via a small metal electrode could lower BP.
Device-Based Therapy in Hypertension Trial
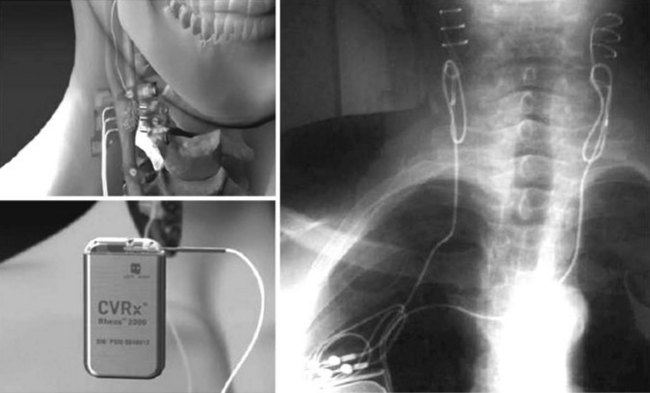
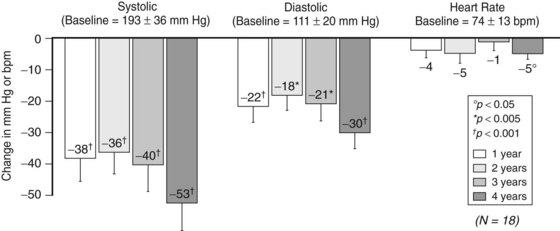
The Device-Based Therapy in Hypertension Trial (DEBuT-HT) was the initial controlled feasibility trial of the commercially available, clinically practical device. Patients with resistant hypertension (systolic BP > 160 mm Hg despite three medications, one of which included a diuretic) underwent bilateral carotid sinus exposure and lead placement. Electrodes were tunneled subcutaneously and attached to a pulse generator implanted in the chest wall (Figure 62-5). Forty-five patients were enrolled in this trial, which was performed in Europe starting in 2006. Eighteen patients have completed 4 years of therapy, and the results of this cohort were recently presented at the 2010 European Society of Hypertension’s twentieth annual meeting in Oslo, Norway, and published in abstract form.14 Blood pressure response has been sustained, and reductions are impressive: mean reduction in systolic pressure is 53 ± 9 mm Hg, diastolic pressure is reduced 30 ± 6 mm Hg, and heart rate is reduced 5 ± 2 beats/min (Figure 62-6). Encouragingly, patients have been able to decrease their antihypertensive medications by approximately one third (5 ± 1.3 medications at onset to 3.4 ± 1 medications at 4 years). DEBuT-HT, which continues to follow patients over time, confirms that this system is clinically practical and shows an impressive, sustained effect.
Rheos Feasibility
Formal testing of the Rheos System (CVRx, Inc, Minneapolis, Minn.) began in 2006. A total of 16 patients (10 in the United States) were implanted in the phase II feasibility trial. The trial was primarily focused on safety and feasibility, which were both shown to be acceptable.12 Results have generally been combined with those from the DEBuT-HT and the Rheos Pivotal Trial as appropriate; however, after 12 months of therapy, mean systolic ambulatory pressures have fallen from 171 to 157 mm Hg, and patients spend 20% more time with systolic BPs less than 140 mm Hg.15 No injury to or midterm abnormalities of the carotid arteries have been identified.16
Rheos Pivotal Trial
The Rheos Pivotal Trial is a phase III prospective randomized trial approved by the U.S. Food and Drug Administration (FDA) and designed to prove efficacy in a blinded, randomized, controlled fashion. The device itself, implantation technique, and trial eligibility (systolic BP greater than 160 mm Hg despite three medications, one being a diuretic) are the same as for the DEBuT-HT and the Rheos Feasibility trial. Patients undergo implantation, and the device is turned off for 4 weeks to allow healing. Next, patients are randomized in a 2 : 1 ratio (study sites and investigators are blinded) to “on” or “off ” for the next 6 months, then all patients are “on” for months 7 to 12. The major study endpoints are differences in BP after the 6-month randomization period between those receiving therapy and those whose devices are off, an improvement in BP at 12 months in patients whose devices had been off, and an overall improvement at 12 months versus enrollment baseline in the entire cohort.17
Enrollment was completed in late 2009, with 322 patients treated (55 roll in, 265 randomized). Unblinding occurred in late 2010, and the results have recently been published.18 Initially “on” and “off ” groups were similar at baseline, having mean systolic BPs of 179 ± 22 and 176 ± 22 mm Hg, respectively, diastolic BPs greater than 100 mm Hg despite taking an average of 5.2 ± 2 medications. After initial discussions with the FDA, endpoints had been decided to be the percentage of patients achieving a response of 10 mm Hg or greater, rather than the absolute BP response. After the first 6 months, 54% of the “on” group showed such a response; however, 46% of the “off ” group did as well, illustrating the interaction of the Hawthorne effect combined with a fairly small goal. This difference, at 7.7%, fell below the a priori target of a 20% response rate; however, after 12 months of therapy (all patients being “on” for at least 6 months), sustained improvement was seen in 88% of patients, well above the a priori target of 65% (p < 0.001). Six months of device therapy led to a 40% reduction in hypertensive crises and a 23% reduction in overall hypertension-associated adverse events (p < 0.001). Implantation and therapy were well tolerated, with a 4.4% permanent nerve injury (much of it from periincisional numbness) being the most significant complication. Long-term device-related adverse events, at 13%, fell below the a priori target of 28% (p < 0.001). A manuscript describing these results is currently (mid-2011) in the review process.
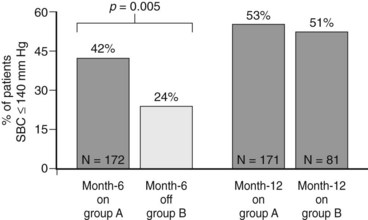
Interestingly, absolute BP comparisons are not yet available because the company is complying closely with the reporting criteria agreed upon with the FDA at the start of the trial. However, the percentage of patients whose systolic BP decreased to less than 140 mm Hg is illustrative (Figure 62-7). After the first 6 months, 42% of those receiving stimulation fell below this goal, whereas only 24% of those in the “off” group did so (p < 0.005). At 12 months of therapy, approximately 52% of patients (who started with a mean systolic BP of approximately 178 mm Hg) were below this target. Finally, post hoc analysis shows that mean systolic BP drop at 6 and 12 months is 26 and 35 mm Hg, respectively.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree