Chapter 104
Carotid Artery
Carotid Body Tumors and Other Disorders
Glenn M. La Muraglia, Virendra I. Patel
Based on a chapter in the seventh edition by Johelen Carleton and Karl A. Illig
Carotid Body Tumors
This chapter focuses on carotid body tumors (CBT) and related paracarotid tumors. It also discusses the carotid sinus syndrome, carotid artery kink and coils, Moyamoya disease, and intracranial vascular lesions.
The carotid body is a neural crest cell-derived chemoreceptor located in the medial portion of the carotid bifurcation. These receptors detect changes in oxygen, carbon dioxide, and pH concentration, and are involved in neurogenic physiologic adaptation to changes in these parameters.1 When tumors develop from these cells, they are referred to as extra-adrenal neuroendocrine neoplasms.2–5 In the head and neck, these tumors are derived from cells associated primarily with the parasympathetic autonomic nervous system.6 They have been commonly referred to by a variety of names, including carotid body tumor (CBT), paraganglioma, glomus, or nonchromaffin or chemodectoma tumors. To better define and compare them with other similar neural crest-derived tumors in this and other anatomic locations, they are also referred to as carotid body paragangliomas.3
Paragangliomas are tumors that are derived from neuroendocrine tissues in the paraganglia of nerves and are known to occur in the cervical, thoracic, and abdominal paravertebral spaces, as well in the adrenal medulla where they are commonly referred to as pheochromocytomas.7,8 Outside the adrenal gland, these tumors are thought to be derived from either the parasympathetic or sympathetic system.6,9
The autonomic cell type of origin of the paraganglioma is anatomically based. Those tumors identified in the neck and upper mediastinum are primarily parasympathetic-derived and generally found to be nonsecretor, nonfunctional tumors, whereas those presenting in the lower mediastinum, retroperitoneum, and the adrenal medulla are sympathetic-derived and are generally secretor, functional tumors.7,10 The paragangliomas that are considered to be secretor, functionally active tumors produce and release catecholamines that often are noted to have significant systemic effects.11,12
Epidemiology
Head and neck paragangliomas are very rare tumors that have an incidence of 1 in 30,000 to 1 in 100,000. The CBT is the most common, accounting for 65% to 80% of these tumors, whereas glomus tumors (jugular bulb and tympanic nerve paraganglioma [15%]) and vagal paragangliomas (5%) are less common.10,13,14 A recent review of CBTs identified diagnosis at a mean age of 55 years (range, 18-94 years) and a male-to-female ratio of 1 : 1.9. More tumors (57%) were on the right side, whereas 25% were on the left, 17% were bilateral, only 1 tumor was functional, and 4.3% were malignant.14 Some studies further emphasized the higher prevalence of women affected by these tumors.4,15
Pathogenesis
Accepted risk factors for CBT include conditions that lead to chronic hypoxemia, which include living at high altitudes, smoking, chronic obstructive pulmonary disease, or other respiratory conditions that result in hypoxia.4,14–17 CBTs can occur sporadically, but are also seen as part of syndromes, including Carney’s triad (gastric stromal sarcoma, pulmonary chondroma, and paraganglioma), von Hippel-Lindau’s disease (pheochromocytoma, spinal hemangioblastoma, and paraganglioma), neurofibromatosis type 1, and multiple endocrine neoplasia type 2 and paraganglioma-pheochromocytoma syndrome.7,8,18
Malignancy in CBTs is not determined on a histologic basis because the cellular appearance is relatively uniform, and most tumors have microscopic evidence of capsular invasion. As such, malignancy can only be diagnosed according to clinical behavior of the tumor: local invasion, recurrence, or evidence of metastasis.19,20 These are rare findings because the rate of “clinical malignancy” is reported to be less than 5%.21 Even locally invasive CBTs are not definitely considered malignant, because it is generally accepted that malignancy can only be attributed to them if they have metastasized to non-neuroendocrine tissue: lymph nodes, lung, liver, and skin.22
Anatomy and Pathology
CBTs characteristically splay the carotid bifurcation, and depending on their size, can encapsulate the external or internal carotid artery, or both. The bulk of the tumor is generally located at, and more often, deep to the bifurcation and can extend over the common carotid artery proximal to the bifurcation. Grossly, they are tan-brown in color, round or tear drop in configuration, and are lobulated with an indentation to reflect the configuration of the carotid artery to which they closely adhere (Fig. 104-1C). Along the surface of the tumor and the periadventitia of the carotid arteries, there are a plethora of friable and enlarged vessels that constitute a thin fibrous capsule of the tumor (Fig. 104-1A).23 Microscopically, the tumors reproduce the architecture of the normal carotid body, which is composed of granular epithelioid chief cells and sustentacular supporting cells. These cells form clusters called Zellballen or “cell balls” that are surrounded by highly vascular sinusoids (Fig. 104-2).24,25 These tumors grow quite slowly, with a reported median doubling time of 4.2 years, with large and small tumors growing at a slower rate than intermediate-sized ones,26 whereas malignant tumors have been occasionally found to grow more aggressively.27
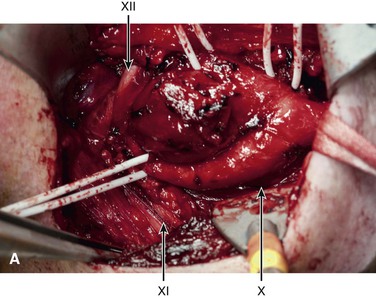
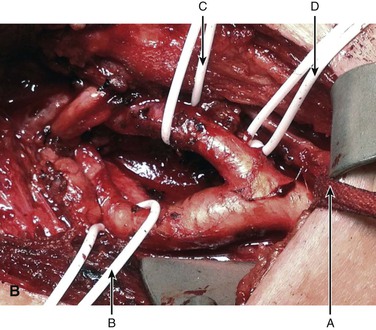
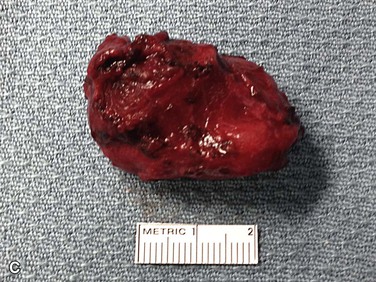
Figure 104-1 Operative photograph of carotid body tumor. A, Dissection of the carotid body tumor with the central carotid body tumor. B, The loop is around the common carotid A (arrow) and vessel loops around the internal carotid artery B (arrow), external carotid artery C (arrow), and superior thyroid D (arrow). The cranial nerves are also identified vagus X (arrow), accessory XI (arrow), and hypoglossal XII (arrow). Postremoval of the tumor noting the splayed configuration of the carotid arteries. C, Gross specimen of the resected carotid body tumor. Note the anterior indentation (right to left) that represents the location of the carotid bulb location when the tumor was in situ.
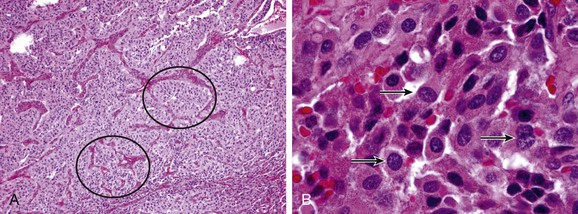
Figure 104-2 Pathology of paraganglioma. A, Histology of carotid paraganglioma, with the characteristic “Zellballen” ball-shaped clusters of cells (circles), 100× magnification. B, Higher power magnification of carotid body tumor cells demonstrating “salt and pepper” chromatin staining characteristic of neuroendocrine tumors, 1000× magnification. (Courtesy Peter M. Sadow, MD)
Genetics
There has been a significant amount of progress since 2000, when a specific mutation was identified in patients with head and neck familial paraganglioma.28 It was a mitochondrial complex II gene mutation that was identified on the enzyme complex of succinate dehydrogenase subunit D (SDHD), an enzyme that plays a role in oxidative phosphorylation of the Krebs cycle. Because other SDH subunit mutations have been identified in other nonadrenal paragangliomas and pheochromocytomas, SDHD identified the comparable genetic basis of these tumors as being from a similar embryologic origin.7,10,18,29 Germ line mutations have been identified to involve the subunits A, B, C, D, and the assembly factor 2 (AF2) of the SDH enzyme complex; the most prevalent ones noted involve the B and D subunits.30,31 It is primarily these mutations that provide the basis for the paraganglioma-pheochromocytoma syndrome, which has five categories (paraganglioma-1 to paraganglioma-5). The mutations of the SDHB are most often found in adrenal, but rarely in the head and neck, paragangliomas, and are the ones most often associated with malignancy.19,32,33 The mutations of the SDHD are most frequently identified in patients with skull base and neck paragangliomas, are rarely malignant, and are often metachronous.34,35
Recommendations for genetic testing for mutations of the SDH subunit genes in patients with head and neck paragangliomas for hereditary paraganglioma-pheochromocytoma syndrome have not been established.18,36,37 Patients with a positive family history, synchronous tumors, young age at onset, recurrent tumors, or evidence of malignancy are considered at particular risk of having a hereditary mutation, and should be strongly considered for genetic testing, whereas others recommend genetic testing in all patients with a paraganglioma.38–41 It has also been proposed that all patients should be screened for SDHB because it has a young age of penetrance, has the highest likelihood of undergoing a malignant transformation, has the poorest long-term prognosis, and can present as an isolated, nonfamilial case.32 Identification of such a genetic mutation in a proband would suggest more aggressive family screening and individual surveillance testing of the affected patients for early detection and treatment.
The finding of a SDHD mutation, which include 30% of hereditary paragangliomas and are present in half of the neck- and skull-based tumors that are identified with paraganglioma-pheochromocytoma syndrome, has clinical implications.42 The presence of the SDHD mutation is a marker that the patient is much more likely to have synchronous paraganglioma lesions than a patient with either another mutation or an isolated case of paraganglioma.13 The recommended genetic testing for neck- and skull-based tumors also suggests evaluations for mutations in SDHD, SDHB, SDHC, and SDHAF2.41
Clinical Findings
CBTs are usually asymptomatic and discovered to be a painless mass or fullness in the neck at the level of the carotid bifurcation.25 In larger tumors, they can be associated with the myriad of presenting symptoms of a space-occupying lesion in this location, such as fullness, pain, dysphagia, odynophagia, hoarseness, and stridor.5,39,43 On physical examination, depending on the size and body habitus of the patient, a fullness or a mass can be palpated in the mid neck at the location of the carotid bifurcation. In thin patients, a palpable mass may be found to be vertically fixed but mobile horizontally (Fontaine’s sign), but similar findings can be caused by other types of neck masses. There is rarely a bruit or a thrill on physical examination.4,15
Although 10% to 22% of patients with CBTs have been historically reported to present with a cranial nerve deficit,4,14 it is less common currently because of improved imaging to identify these tumors; they are identified at an earlier stage and at a smaller size.25 Cranial nerve paresis may reflect nerve compression and is not necessarily diagnostic of a paraganglioma arising from the cranial nerve itself. In addition, vagal paragangliomas may arise from and infiltrate a nerve, but as opposed to neoplastic nerve infiltration, they may not necessarily render the nerve dysfunctional.44,45 It is therefore of paramount importance to preoperatively undertake a careful neurologic examination to determine the integrity of the cranial nerves.
The rare functional CBT may present with symptoms compatible with sympathetic hyperactivity.6,27 These include palpations, hypertension, tachycardia, and headache from the release of catcholeamines.
Diagnostic Evaluation
CBTs are usually identified by clinical examination or found incidentally on imaging studies.25 When suspected, the diagnosis can be easily confirmed by imaging because they have a characteristic anatomic configuration, are hypervascular in nature, and have a high uptake on positron emission tomography (PET). Duplex ultrasound, computed tomography (CT), and magnetic resonance imaging (MRI) are all effective for diagnosis; however, axial imaging, either CT angiography (CTA) or MRI, is the preferred modality for surgical planning of tumor resection because it best defines the relationship of the tumor with the artery bifurcation and the likely location of the cranial nerves.13,46,47
CBTs are well-defined, hypervascular masses that characteristically splay the carotid bifurcation, a characteristic that is pathopneumonic. On duplex ultrasound interrogation, which is ideal to screen a patient for a CBT (Fig. 104-3), these masses appear hypoechoic.47 On CT and MRI, CBTs may appear as somewhat heterogeneous, highly vascular masses, but do not exhibit evidence of invasion of the carotid arteries or adjacent tissues (Fig. 104-4).46,48
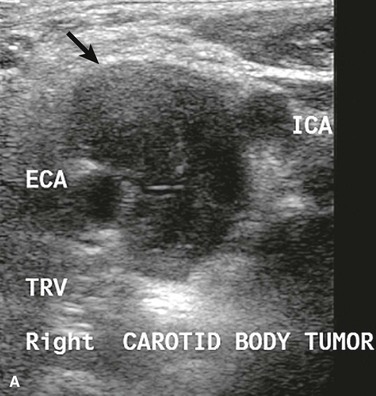
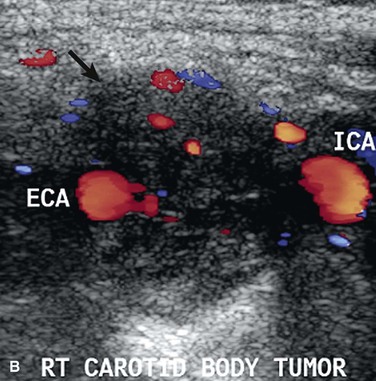
Figure 104-3 Duplex ultrasound of carotid body tumor, transverse images (TRV) depicting the characteristic splaying of the internal (ICA) and external carotid artery (ECA) with the mass of the carotid body tumor (arrow). A, Grey scale, B, Color flow.
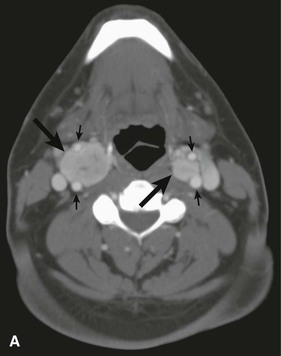
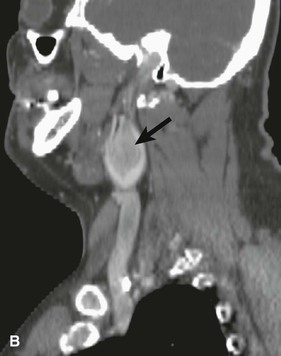
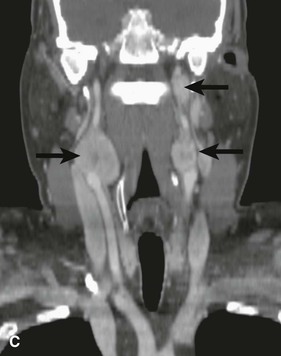
Figure 104-4 CTA of a patient with bilateral carotid tumors and a skull base paraganglioma. A, Axial view tumor (large arrows) demonstrating the characteristic splaying of the internal and external carotid artery (small arrows). B, Sagittal view of one of the carotid body tumors (large arrow) demonstrating the splayed carotid bifurcation. C, Coronal view of the bilateral carotid body tumors and the paraganglioma at the skull base.
Needle biopsy is unnecessary for diagnosis and can be hazardous because often these hypervascular masses will develop bleeding that is sometimes difficult to control.39 Because CBTs can be bilateral and paragangliomas can be multicentric, especially in familial cases, an octreotide scan is recommended for all patients once the diagnosis of a CBT has been made.49 Octreotide scintigraphy, a nuclear medicine body scan that measures the uptake of a radiolabeled somatostatin analogue, is more specific and highly sensitive in the detection of paragangliomas, and is recommended to identify synchronous lesions in the body or other lesions that may not have been detected by neck imaging.49 Some have recommended PET, which is very sensitive to diagnose paragangliomas less than 1 cm in size, but it may be less specific.13
Historically, preoperative angiography was the “gold standard” diagnostic procedure for CBTs and was considered mandatory, both to confirm the diagnosis and to provide accurate preoperative delineation of the vascular supply.50 With improvements in quality and high resolution of noninvasive imaging, angiography is rarely utilized for this purpose in contemporary practice because CT and MRI provide excellent anatomic characterization of CBTs for operative planning (see Fig. 104-4) and obviate the inherent risks associated with invasive angiography of the cerebral circulation.5,14
If there is clinical suspicion for the rare occurrence of a tumor that is functional and secreting catecholamines, biochemical evaluation is recommended.27 This is undertaken with a plasma or a 24-hour urinary collection for metanephrines and the catecholamines, epinephrine, and norepinephrine, with the former being the more sensitive test.53 Should this prove positive, the information greatly assists the administration of a safe anesthetic for the surgical resection.
Carotid Body Tumor Embolization
Intraarterial Embolization
Embolization of the blood supply to CBTs has been advocated by some to reduce intraoperative bleeding during resection of large tumors. This technique uses an intraarterial catheter to inject embolic microspheres, and occasionally, occlusive coils into branches of the external carotid artery, which have been proven to provide blood supply to the tumor.
Embolization of CBTs was first described in 1983.51 It was reported to decrease tumor size and reduce tumor vascularity, thereby facilitating surgical resection by creating a less bloody operative field (Fig. 104-5).23 Since that time, the role of preoperative embolization to facilitate resection of CBTs has been controversial. Although several retrospective studies demonstrated no difference in blood loss or perioperative morbidity between embolized and nonembolized groups,5,52 two others found reduced intraoperative bleeding after embolization of tumors of more than 3 cm in diameter (but not for smaller CBTs).23,53 Currently, there are no prospective studies to validate the benefit of preoperative CBT embolization to minimize blood loss or decrease postoperative complications.
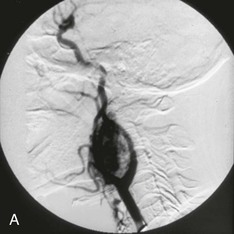
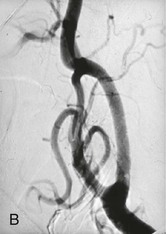
Figure 104-5 Arteriogram performed of a carotid body tumor at the (A) beginning of and at the (B) completion of carotid body tumor embolization. Please note the paucity of blood flow to the tumor after the embolization.
There are concerns, however, about the risk of stroke during embolization of CBTs from either reflux of the embolic material or thromboembolism into the cerebral or ophthalmic circulation directly from the catheter manipulation. One series of patients with CBTs treated with preoperative embolization reported a stroke rate of 1 in 6 patients, another reported 1 in 11 patients, and a third 0 in 33 patients, suggesting that high volume in this very specialized technique yields better outcomes.5,23,25 This technique uses polyvinyl alcohol microspheres and occasionally microcoils to achieve preoperative embolization of both the micro and macro circulations of the CBT, and is a specialized technique requiring neuroradiology expertise. It may be indicated in clinically challenging situations with large CBTs or in difficult to access anatomic locations, but is likely not used in routine cases. It should be noted that when preoperative embolization is undertaken, expeditious surgical resection should be performed, preferably within 24 hours and no later than 48 hours, in an effort to avoid additional surgical challenges from a postembolization inflammatory response.23
Direct Embolization
As opposed to intraarterial embolization, there have been case reports of patients treated with intralesional embolization directly into the tumor. One European report utilized poloxamer 407, a reverse thermal polymer that forms a gel at body temperature, which was embolized intraoperatively directly into the tumor, resulting in successful devascularization.54 Another report used direct intralesional Onyx (Covidien, Plymouth, Massachusetts) embolization preoperatively; however, this resulted in multiple cranial nerve and cervical sympathetic plexus injury from local injury.55 There have also been reports of using covered stents to exclude the external carotid artery to diminish blood flow to the tumors to facilitate surgical resection.56,57 However, this approach does not address collateral filling of the vessels that supply the CBT, and the long-term natural history of a covered stent in the carotid artery is unknown.
Surgical Treatment
Complete surgical resection is the preferred treatment of CBTs. The two major challenges in the surgical removal of CBTs are the preservation of adjacent cranial nerves and maintaining the integrity of the carotid arteries. It is for this reason that we advocated utilizing a multidisciplinary team approach, particularly for large CBTs, with a head and neck surgeon skilled in identification of distorted cranial nerve anatomy and a vascular surgeon skilled in carotid dissection and reconstruction, should it be necessary.23
Avoiding Cranial Nerve Injury
There is a high incidence of permanent cranial nerve complications, even in contemporary series of CBT resection, that range from 8% to 12%.5,43 Cranial nerves can be injured either by the retraction of the surrounding tissue or the dissection of the tumor mass in a bloody field. Therefore, it is optimal to expose the vagus (X), its superior laryngeal branch when appropriate, spinal accessory (XI), and hypoglossal (XII) nerves before the tumor is dissected from the carotid artery (see Fig. 104-1). In addition to these primary cranial nerves, there are two other nerves that need to be considered during the surgical procedure: the marginal mandibular branch of the facial nerve and the cervical sympathetic plexus. The former can be injured by deep retraction on the mandible and can result in weakness of the labial muscles. The latter can be injured in the posterior dissection of the tumor in the prevertebral and paraparyngeal tissues, and can result in Horner’s syndrome.
Carotid Body Tumor Classification
Shamblin et al24 introduced a classification scheme that reflects the degree of technical challenge in tumor excision. Type I tumors are small lesions nested in the carotid bifurcation, and can generally be removed without difficulty (Fig. 104-6). Type II tumors are larger, significantly splay the carotid bifurcation, but do not circumferentially encase the carotid arteries. Type III tumors are large, encapsulate the internal and/or external carotid arteries, and often adhere or incorporate the adjacent cranial nerves. These are the most surgically challenging, may benefit from preoperative tumor embolization, occasionally require division of the external carotid artery to facilitate tumor resection, and rarely require extirpation of the internal carotid artery with the tumor followed by carotid interposition reconstruction.
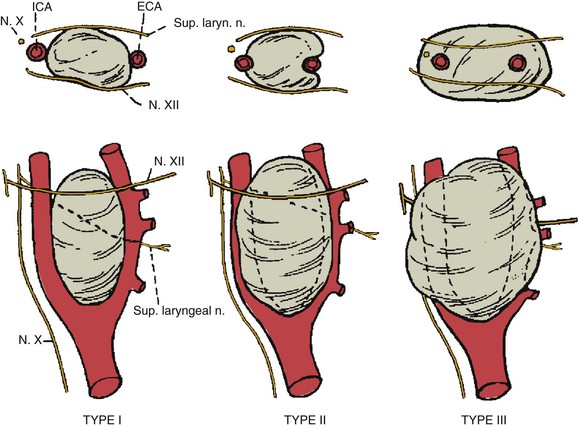
Figure 104-6 Shamblin’s classification of carotid body tumors based on the degree of difficulty of resection. Type I tumors are localized and easily resected. Type II tumors include tumors adherent to or partially surrounding vessels. Type III tumors intimately surround or encase the vessels and nerves. ECA, External carotid artery; ICA, internal carotid artery. (Redrawn from Hallet JW: Trends in neurovascular complications of surgical management for carotid body and cervical paragangliomas: a fifty-year experience with 153 tumors. J Vasc Surg 7:284-291, 1988.)
Operative Technique
There are also a number of general operative considerations for CBT resections. Although regional anesthesia can be considered for the smaller tumor excisions, general anesthesia is preferred, particularly for difficult cases. We recommend neuromonitoring with electroencephalography for the rare instance of carotid clamping for carotid reconstruction.23 It is advisable to include the medial thigh in the operative field for saphenous vein access if artery resection is possibly required, although prosthetic patches or grafts may also be used. In circumstances when the dissection is high or difficult, patients should be nasally intubated to provide additional exposure, and consideration should be given to anterior subluxation of the mandible to even further facilitate exposure.58
The patient is positioned similar to a carotid endarterectomy, in the supine position with the neck rotated away from the surgical field. Use of a posterior thyroid inflation bag is optional, depending on the patient’s neck configuration. A number of surgical incisions have been advocated, including a curvilinear incision centered on the mid portion of the tumor, an incision along the anterior border of the sternocleidomastoid muscle, and in cases of high, large tumors, a modified radical neck T incision has been described to facilitate the exposure of large tumors (Fig. 104-7).4
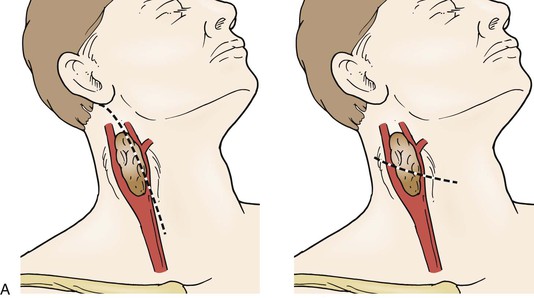
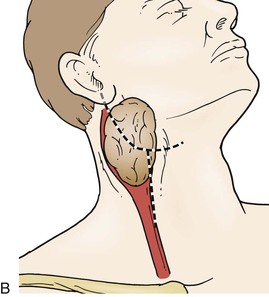
Figure 104-7 Options for neck incisions for carotid body tumor resection: for smaller tumors there are two options. A, A longitudinal incision anterior to the sternocleidomastoid muscle, the superior portion curving posteriorly to the mastoid, if necessary. A curvilinear incision along the midportion of the tumor along the skin folds of the neck. B, For larger tumors and those with a high or deep extent, a modified radical T incision can provide more extensive exposure.
As the carotid sheath is entered, one should identify and expose the vagus (X) and its superior laryngeal branch, the accessory (XI), and the hypoglossa (XII) cranial nerves before tumor resection. During this portion of the procedure, the lymphatic tissue is dissected off the anterior border of the tumor to expose the carotid arteries and tumor mass beyond the bifurcation. Fixed retraction devices can then be readily placed to optimize the exposure for the CBT without inadvertently compressing a cranial nerve. Initially, exposure should be obtained proximally at the common carotid artery to encircle it with a Rummel tourniquet, if there is a subsequent need for carotid reconstruction with a carotid shunt. The dissection should then be performed along the lateral borders of the internal and external carotid arteries to define the edge of the tumor and divide any discrete blood supply that can be identified from the external carotid artery. Distal control is subsequently obtained by placing vessel loops around the internal and external carotid arteries beyond the tumor mass. Because the CBT is often densely adherent to the wall of the carotid artery, the artery can be easily injured in the dissection of the tumor mass from the carotid bifurcation. As the carotid arteries have been splayed with tumor growth, the artery wall has been stretched and may be attenuated, leaving the carotid artery more prone to injury and perforation than in surgery for carotid occlusive disease or during resection of cervical malignancies. For this reason, periadventitial sharp dissection rather than blunt dissection is indicated to avoid arterial wall injury or perforation. Careful coagulation using bipolar forceps and thrombin impregnated gelfoam are adjuncts that can facilitate hemostasis. As the internal and external carotid arteries are dissected free, the tumor is mobilized circumferentially first at the inferior margin, where it is generally most adherent to the carotid wall at the bifurcation. Identification of the ascending pharyngeal artery, with suture ligation of its origin on the external carotid artery, facilitates the procedure and reduces blood loss. Posterior dissection of the CBT needs to be carefully performed to avoid injury to the superior laryngeal nerve and the cervical sympathetic plexus. Occasionally, the external carotid artery needs to be divided to facilitate exposure and removal of the tumor mass off the carotid bifurcation. Because this is generally well tolerated, the external carotid artery does not need to be bypassed or re-implanted. If the tumor cannot be adequately resected off the internal or common carotid artery, a portion of the artery may need to be removed en bloc, followed by appropriate reconstruction utilizing saphenous vein or prosthetic material.
Surgical Results
Historically, CBT surgery has been associated with significant morbidity and mortality. The highest complication rate involves cranial nerve injury, some of which resolve over time. Contemporary results have significantly improved outcomes with reported mortality rates between 0% and 3%, and stroke rates between 0% and 8%. However, cranial nerve injury remains high, with two large contemporary series reporting a permanent deficit of 9% and 3%, respectively.5,14 Patients with paragangliomas involving the vagus nerve or other cranial nerves will nearly always have complete loss of nerve function because it encapsulates the nerve that needs to be resected. Other reported complications include the baroreflex failure syndrome after bilateral CBT resection,59 and the development of a psuedoaneurysm,60 a rare event that underscores the utility of checking the carotid duplex in the postoperative period.
Other Treatment
Due to their indolent nature, the decision to treat or to observe patients with CBTs is dependent on multiple factors, such as tumor size, age of the patient, and associated comorbidities.26,53 As such, observation may be a viable option in patients with small tumors or poor life expectancy.
Another option for selected patients is radiation therapy.61,62 Although surgery is the treatment of choice for CBTs, radiation therapy has primarily been used for the treatment of patients who are not operative candidates and in patients who develop recurrent cervical paragangliomas, although some consider surgery to be the preferred treatment of recurrent CBTs.63 Radiation therapy has obtained acceptable local control of tumors,62 but there are no prospective comparative studies between surgery and radiation therapy for the treatment of CBTs.
Other Primary Head and Neck Paracarotid Tumors
Glomus Jugulare and Glomus Tympanicum
Glomus jugulare are paragangliomas that arise at the base of the skull at the jugular foramen or at the temporal bone along the tympanic canal (Fig. 104-4C). Although there are a number of other tumors that can be located at the skull base, the most common that are identified are the paragangliomas. Because of the anatomic location and hypervascularity of these lesions and the proximity to multiple cranial nerves, the jugular vein, and the carotid artery, their surgical resection has been a major challenge that is accompanied by much higher morbidity and mortality than CBT resection.64 Because the complications can be quite significant, including dysphagia, aspiration, and meningitis from cerebrospinal fluid leak, other treatment modalities have been carefully evaluated for their treatment, including radiotherapy.65,66
Fractionated radiotherapy was utilized with mixed success because there were significant morbid side effects to the adjacent tissue, including bone and brainstem necrosis, stroke, and severe dry mouth.67,68 With the reduction of the radiation field and the advent of radiosurgery and sterotactically guided radiation, the complication rate dropped significantly, and there was good local tumor control in the short term.61,62 Although there was some improvement of symptoms with radiosurgery, such as those associated with nerve compression, a significant shrinkage of the paraganglioma was not observed.69 The success of sterotactic radiation does not negate the benefits of microsurgery in some patients, because some paragangliomas require surgical resection, especially in the presence of compressive symptoms.70,71 Together with radiosurgery, microsurgery for these lesions are complementary in the treatment algorithm of these challenging tumors. Each patient needs to be carefully considered for each or a combination of these treatment modalities, depending on their presentation.66,71,72
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


