Chapter 101
Carotid Artery
Stenting
Piergiorgio Cao, Paola De Rango
In 1977 and 1980, Mathias et al1,2 and Kerber et al3 reported successful results with percutaneous angioplasty for carotid stenosis using technology derived from peripheral arterial angioplasty. Balloon-expandable stents were first deployed in the carotid artery in 1989 but were prone to extrinsic compression and had a high rate of major adverse events.4–6 These issues were resolved with self-expanding mesh wire Elgiloy carotid stents and, later, nitinol stents.7 Nevertheless, the high likelihood of embolic stroke during carotid stent deployment was a major concern that limited the early enthusiasm for endovascular treatment of carotid stenosis. It was with the introduction of embolic protection devices (EPDs) in 19908 that carotid artery stenting (CAS) developed rapidly and reached widespread expansion in the subsequent years. The feasibility of CAS to treat carotid stenosis outside surgical settings has accelerated the use of the procedure as an alternative to surgery for treatment of patients with carotid stenosis. Proponents of CAS have stressed the apparent simplicity, quickness, and comfort of the new, less invasive procedure compared with carotid endarterectomy (CEA). However, the procedure has undergone large scrutiny in the last 15 years,9–41 and today there are still concerns about the consideration of CAS as an “equivalent” therapeutic option to CEA in most patients with carotid stenosis because of increased periprocedural stroke risks and costs.9,27 After the publication of a number of randomized clinical trials (RCTs),10–22,28–37 multiple meta-analyses,42–49 and several large registries and worldwide series,50–109 there are data to support a twofold higher perioperative stroke risk with CAS compared with CEA when it is performed in unselected patients with symptomatic carotid stenosis. Currently, with better patient and operator selection and improved technology, CAS may be considered an alternative to CEA only in certain subgroups of patients.
Patient Selection
The incidence of complications of CAS strongly depends on how patients are selected and on the presence of factors that are associated with increased periprocedural risks. Better patient subgroup selection is associated with notably decreased CAS complications. However, most data for subgroup analyses are provided by smaller samples not powered to assess treatment differences and should be interpreted with caution. The detailed decision-making process to select CAS, CEA, or medical management in patients with carotid stenosis is developed in Chapter 99.
Clinical Considerations
Age
Advanced age is one of the most commonly used factors in surgical risk stratification. Given the less invasiveness and avoidance of general anesthesia, CAS was initially considered a promising alternative to CEA, especially in elderly patients. Today, there is compelling information supporting CEA rather than CAS in the treatment of old patients, particularly octogenarians.20,31,110,111
In a post hoc analysis of the SPACE trial, it was found that age (≥68 years) was significantly associated with the risk of stroke and death in the CAS group (P = .001) but not in the CEA group (P = .534).20 Similarly, CREST showed that older age increases the risk for the combined endpoint of stroke/death or myocardial infarction (MI) for CAS (P = .02), with the efficacy of CAS and CEA approximately equal at the age of 70 years.31 CAS risk for the primary endpoint increased with age (P < .0001) by 1.77 times (95% confidence interval [CI], 1.38-2.28) per 10-year increment, whereas there was no evidence of increased risk for CEA (P = .27). The treatment-by-age interaction for CAS and CEA was not altered by symptomatic status (P = .96).31
Pooled analysis of the three European RCTs (EVA-3S, SPACE, and ICSS) from the Carotid Stenting Trialists’ Collaboration found that in patients 70 years or older, there was a twofold increase in the 120-day stroke or death risk with CAS over CEA: 12.0% versus 5.9% (risk ratio [RR], 2.04; 95% CI, 1.48-2.82; P = .0053). For patients younger than 70 years, the risk was comparable: 5.8% in CAS and 5.7% in CEA (RR, 1.00; 95% CI, 0.68 to 1.47).110
In the Cochrane meta-analysis of pooled RCTs, the odds ratio (OR) of CAS versus CEA for 30-day death or any stroke risk was 1.16 (95% CI, 0.80-1.67) in patients younger than 70 years and 2.20 (95% CI, 1.47-3.29) in patients 70 years or older.42
The Society for Vascular Surgery Vascular Registry stratified outcomes of CAS and CEA by age and compared the composite outcome of death, stroke, or MI at 30 days among 1347 CEA and 861 CAS patients younger than 65 years and 4169 CEA and 2536 CAS patients 65 years and older. In patients 65 years and older, CEA had lower death (0.91% vs. 1.97%; P < .01), stroke (2.52% vs. 4.89%; P < .01), and composite death/stroke/MI (4.27% vs. 7.14%; P < .01) rates.80 Significant difference in composite outcome for old patients persisted when the analysis was performed separately in symptomatic (5.27% vs. 9.52%; P < .01) and asymptomatic (3.31% vs. 5.27%; P < .01) subgroups.80
Last, in-hospital data derived from a large number of carotid procedures (495,331) retrieved through the Nationwide Inpatient Sample (NIS) showed age 70 years and older to be an important predictor of postoperative stroke (OR, 1.7; 95% CI, 1.2-2.5; P < .0025) and cardiac complications (OR, 1.3; 95% CI, 1.0-1.6; P < .045) after CAS.111
Although CAS is a less invasive approach, older patients frequently have extensive atherosclerotic diseases that translate into more frequent occurrence of multiple embolic sources, adverse conditions for endovascular approach (such as diffuse calcification or “shaggy” aortic arches), and impaired cerebrovascular reserve with higher sensitivity to minor cerebral emboli damage. Manipulations with wires, catheters, and sheaths in tortuous or diseased arteries proximal to the target lesion can contribute to the increased hazards of CAS in older patients.
Sex
Sex-specific differences in cardiovascular disease and CEA have been well described. However, whether such disparities apply to CAS remains uncertain as randomized and nonrandomized studies report conflicting results.33,48,112–115
A secondary analysis of the CREST trial suggested that periprocedural risk of stroke/death appeared to be higher in women who had CAS than in those who had CEA: 5.5% versus 2.2% (P = .013).33 The difference in rates was more evident in symptomatic women (7.5% vs. 2.7% in CAS vs. CEA, respectively; P = .030), whereas it was not confirmed in asymptomatic women (P = .28).33
In the meta-analysis of the three European RCTs (Carotid Stenting Trialists’ Collaboration), an opposite trend was shown; a lower CAS to CEA risk ratio in women than in men (1.22 vs. 1.68) was assessed at 120 days.48 A further meta-analysis including CREST and CAVATAS was consistent with the not real effect of sex on the risks of CAS or CEA.112
Outside RCTs, there is conflicting information in reporting the effect of sex on CAS outcome.113–117 The potential and uncertain higher stroke risk in symptomatic women after CAS may be related to greater embolic potential in female carotid plaque.117 This hypothesis needs to be substantiated by more data.
Statewide risk-adjusted analysis of 18,320 women undergoing CEA and 2293 undergoing CAS found that the risk of in-hospital stroke/death was 1.7-fold higher in symptomatic and 3.4-fold higher in asymptomatic women receiving CAS compared with CEA.115
The ALKK-CAS registry showed that for the group of 1443 women, the total composite in-hospital endpoint of nonfatal stroke and death was comparable to that of men: 3.0% versus 3.4%. Despite the older age of women, all in-hospital outcomes of CAS in women were comparable to those of men in both symptomatic and asymptomatic patients.116
Currently, sex cannot be considered among high-risk criteria for CAS.
Timing in Symptomatic Patients
In recent years, there is evidence recommending treatment with “early” CEA in symptomatic patients to prevent the risk of recurrent stroke, which is four to five times higher in the early period after the onset of symptoms.118 Whereas for patients undergoing CEA there is increased benefit from early surgery performed within the first 7 days of the symptomatic event, the same data are conflicting when they are applied to CAS.119–122 The Carotid Stenting Trialists’ Collaboration meta-analysis of the three European RCTs observed that patients undergoing CAS within 14 days of their most recent symptom were almost three times more likely to have a procedural stroke or death (8.6% vs. 3.2%; OR, 2.7; 95% CI, 1.36-5.45; P = .30) than patients undergoing CEA.48 Updated data from the same group recently showed that the stroke risk in CAS compared with CEA appeared to be greatest in patients treated within 7 days of symptoms. Patients treated with CAS in this period had 9.4% risk of periprocedural stroke/death compared with 2.8% for CEA, leading to more than threefold increased risk of CAS versus CEA adjusted for age, sex, and type of event (hazard ratio [HR], 3.4; 95% CI, 1.01-11.8).119
Analysis of 482 symptomatic patients included in the CAPTURE registry showed that an interval of 0 to 13 days from symptoms (stroke or transient ischemic attack) to CAS was an independent predictor of adverse 30-day outcome (OR, 2.52; 95% CI, 1.33-4.78; P = .0047).63 Topakian et al120 analyzed the effect of timing in 77 CAS patients and identified old age and treatment within 2 weeks as the only predictors of increased risk for 30-day complications; stroke and death rate was 26% versus 1.9% in those treated later.
Conversely, in a cohort of 320 patients from two centers described by Gröschel et al,121 the 30-day complication rate after CAS was 8.45%, but early stenting (<2 weeks) was not associated with an increased risk of stroke.
Lin et al,122 using a single-center prospective registry with 224 CAS procedures in symptomatic patients, found that the risk of periprocedural stroke was 3.45% in the early (<4 weeks) and 5.95% in the late (>4 weeks) intervention groups (P = .5). The 30-day stroke/death and MI rates were also similar (6.03% vs. 8.33%).
It is intuitive that endovascular manipulation of a carotid lesion recently symptomatic would have increased embolic risk based on knowledge of carotid plaque pathology as reviewed in Chapter 97. Nevertheless, management of patients by CAS in the early period after the onset of a transient ischemic attack or minor stroke is a challenge to be solved by larger prospective studies focused on timing.
Asymptomatic Carotid Stenosis
Data from CREST and SAPPHIRE suggest that in properly selected asymptomatic patients, CAS, performed by experienced interventionalists, may be equivalent to CEA. Nevertheless, these findings must be interpreted in light of recent studies and guidelines suggesting that for many asymptomatic patients, especially those with short life expectancy or with recurrent stenosis more than true carotid plaque (like many of those included in SAPPHIRE), best medical therapy may be the optimal treatment modality.123–128 Currently, there are insufficient data for CAS to be recommended as a primary therapy for asymptomatic patients with severe carotid stenosis. RCTs are ongoing.38–41
Combined Carotid and Coronary Artery Disease
Because the incidence of concomitant carotid and coronary atherosclerosis is significant and presence of carotid disease has been associated with an increase in the incidence of stroke in the perioperative period for cardiac surgery, management of concomitant carotid and coronary disease has been the subject of considerable debate. The use of CAS would be a logical alternative to CEA in this setting because of its less invasiveness. However, data are not sufficiently robust to support definitive recommendations.123–129 A review of the NIS found that the risk of perioperative stroke was 62% greater in patients undergoing CEA plus coronary artery bypass grafting (CABG) than in those undergoing staged CAS before CABG (OR, 1.62; 95% CI, 1.1-2.5; P = .02). Nevertheless, rates of in-hospital mortality were similar with the two approaches (5.2% vs. 5.4%).130
A meta-analysis of published studies of CAS plus CABG by Naylor et al131 showed a stroke/death rate at 30 days of 9.1%. Although CAS was suggested as a valuable alternative in combined carotid and coronary operations, the stroke/death risks remained questionable because most carotid stenoses treated were asymptomatic.131 Whether the low rate of complications with CAS plus CABG shown in other small studies132–134 reflects case selection bias or an intrinsic safety advantage remains uncertain and should be clarified in properly designed prospective studies.
One of the most challenging issues to be solved is that catheter-based carotid interventions require treatment with a potent platelet inhibitor such as clopidogrel and dual antiplatelet therapy, which can increase the risk of major bleeding associated with cardiac surgery. Conversely, delay of antiplatelet therapy raises the risk of stent thrombosis and stroke.
Anatomic Features
Several anatomic factors may influence the outcome of CAS. Recent analysis from the EVA-3S trial found that the risk of stroke or death in CAS was higher in patients with internal carotid artery (ICA)–common carotid artery (CCA) angulation of 60 degrees or more (RR, 4.96; 95% CI, 2.29-10.74).135 A systematic review of literature by the same EVA-3S authors including 56 studies and 34,398 patients with CAS confirmed that the risk of 30-day stroke/death was higher in patients with increased carotid vessel angulation (RR, 3.41; 95% CI, 1.52-7.63) and when the target ICA lesion was more than 10 mm in length (RR, 2.36; 95% CI, 1.28-3.38). There was no significant increase in risk of stroke/death in patients with type III aortic arch, arch calcification, ostial involvement, calcification, ulceration, degree of stenosis, or contralateral carotid occlusion as also suggested by Sayeed.135,136
A similar analysis of CREST on morphology predictors of outcomes showed that lesion length, eccentric lesions, ulcerated lesions, percentage stenosis, and procedural time were all potential factors not contributing to the age-related risk differences in the CAS treatment group.31
Preprocedural assessment of carotid stenosis is generally based on duplex ultrasound, which provides morphologic and hemodynamic information at the extracranial carotid level. However, in patients with diffuse vascular disease, it is advisable to obtain full supra-aortic vessel imaging with magnetic resonance angiography or computed tomographic angiography before scheduling intervention with CAS. This allows careful evaluation of the aortic arch, the origin and possible tortuosity of all supra-aortic trunks, and the severity of stenosis. In addition, computed tomographic angiography can provide information on the presence and extent of calcification and the morphology of the plaque, and it can make accurate measurements of the diameter of the CCA and ICA for selection of the devices to be used.
Aortic Arch
Factors involving the aortic arch that may increase technical complexity during CAS include the presence of extensive aortic wall irregularities with multiple atheromas (shaggy aorta) and severe aortic calcification (eggshell aorta). A patient with a shaggy aorta may have an absolute contraindication to CAS from the high risk of catastrophic distal embolism not only to the brain but, rarely, to the viscera and lower extremities. An eggshell aorta is associated too with the risk for intimal disruption and embolism because of lack of compliance while the guide wires, catheters, and sheaths are being directed. Last, the stiffness of the vessels may decrease the torqueability of wires and catheters and cause resistance to progression of the sheath or stent delivery system to the target lesion.
Arch morphology can be variable and becomes more elongated and tortuous with advancing age. The upper inner aspect of the arch becomes a fulcrum; with increasing tortuosity, the ascending aorta and the transverse arch can elongate and push the aortic valve and the origins of the innominate artery and left CCA downward. As the arch becomes more tortuous, the origins of the major branches become more difficult to negotiate by remote endoluminal access.
The shape and curvature of the aortic arch can be categorized into three types, depending on the position of the innominate artery as defined by two horizontal lines drawn across the highest point of the outer and inner curvatures of the arch. In a type I aortic arch, the great vessels arise above or in the same horizontal plane as the outer curvature of the arch. In a type II aortic arch, the origin of the innominate artery lies between the horizontal planes of the outer and inner curvatures of the aortic arch. In a type III arch, the innominate artery lies below the horizontal plane of the inner curvature of the arch (Fig. 101-1). The more inferior the origin of the supra-aortic vessels (type II or III arch), the greater the difficulty in gaining access to the carotid arteries and catheter guidance and exchange. A type III aortic arch may lead to prolonged catheter manipulation with a possible risk for aortic plaque embolization.
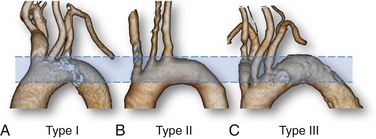
Figure 101-1 Aortic arch classification. A, Type I: the great vessels arise above or in the same horizontal plane of the outer curvature of the arch. B, Type II: the origin of the innominate artery lies between the horizontal planes of the outer and inner curvatures of the aortic arch. C, Type III: the innominate artery lies below the horizontal plane of the inner curvature of the arch.
In addition to these arch configurations, some congenital variations in the origin of the great vessels are relatively common. The so-called bovine arch, present in up to 27% of patients,137 may take two different anatomic patterns. In the more frequent type 2 (8% to 10% of cases), the innominate artery and left CCA share a common origin (Fig. 101-2); in the other type, the common carotid branch takes off from the innominate artery. A “pure” bovine arch, extremely rare, occurs when there is a common brachiocephalic arterial trunk originating from the arch that gives rise to both subclavian arteries (right and left) and a single bicarotid trunk. The bicarotid trunk then branches into two separate trunks, left and right CCA.137
Carotid Tortuosity and Calcification
Heavy circumferential calcification, especially if it is associated with tortuosity along the carotid vessels, increases the difficulty of accessing the lesion. Severe distal ICA kinking or coiling may prevent positioning of the distal EPD with a landing zone sufficient for stent deployment and may predispose to vascular spasm at the end of the procedure. When the angulation is located at the distal end of carotid plaque, stent deployment may change the conformation of the vessel and cause flow-limiting angulation. On occasion, extreme tortuosity may preclude a patient from undergoing intervention.
Finally, extensive calcification in the area of the stenosis may make stent delivery more difficult and may lead to insufficient expansion after deployment and recoiling of the dilated stenosis after deflation of the balloon.
Plaque Morphology
Quantitative and qualitative carotid plaque analysis, primarily achieved with duplex ultrasound, has been suggested as an important parameter for assessing the risk of stroke during CAS.138–142 Details of carotid duplex ultrasound are provided in Chapter 98. There appears to be a higher potential for embolism during CAS in the presence of hypoechoic or echolucent soft plaque.139–142 However, the definition of plaque echogenicity is operator dependent and difficult to classify in terms of different grades of severity. Novel computerized ultrasound technology, such as the gray scale median score, has been proposed141; however, its validation and reproducibility are not universally accepted for routine use in clinical practice.143 In general, the reliability of ultrasound evaluation and correlation with pathologic specimens remain under study, and the clinical applicability of these observations awaits solid confirmation.
Contralateral Carotid Occlusion
There are conflicting data in the literature supporting the increased risks of stroke for patients with contralateral carotid occlusion.144–147 The ALKK-CAS Registry showed overall major stroke and death rates in patients undergoing CAS comparable to those of patients without contralateral carotid occlusion.144 Similar information was provided by the Illinois experience, which found only 2.6% periprocedural stroke in CAS patients with contralateral carotid occlusion.145 Brewster et al,147 in a small prospective study, found two transient ischemic attacks and no perioperative stroke in the group of CAS patients with contralateral carotid occlusion, and 30-day and midterm outcomes were comparable to those of patients without contralateral carotid occlusion.
Currently, the observed outcomes do not support use of contralateral carotid occlusion as a selection criterion for CAS over CEA in the absence of other indications.
Adverse Neck Conditions
CAS is preferred to CEA in patients with local factors that increase complexity of CEA, such as tracheostomy, prior contralateral nerve palsy, lesions that extend proximally near to the clavicle or distally to the C2 vertebral body, and situations in which local tissues are scarred and fibrotic from prior ipsilateral surgery or external beam radiotherapy.123–127 A meta-analysis of 27 articles including 361 CAS and 172 CEA procedures performed for carotid stenosis after radiation therapy showed perioperative risks for any cerebrovascular events of 3.9% with CAS and 3.5% with CEA. However, risk of cranial nerve injury was 0% after CAS and 9.2% after CEA, whereas CAS was followed by higher risk of restenosis over time.148 Another systematic review including 211 surgical procedures and 510 CAS procedures for post-radiotherapy carotid stenosis showed 10.3% cranial nerve palsy occurrence (0.6% permanent) in the surgical group. There were no statistically significant differences in 30-day rates of stroke/death between surgery and CAS in both symptomatic (OR, 0.52; 95% CI, 0.14-1.98; P = .38) and asymptomatic (OR, 0.55; 95% CI, 0.06-5.42; P = .99) patients.149
A complete outline for decision making based on clinical and anatomic criteria to select CAS, CEA, or medical management in patients with carotid stenosis is described in Chapter 99.
Periprocedural Medical Management
Adequate medical management involves intraprocedural therapy and treatment in preparation for or after CAS.
Intraprocedural Therapy
Adequate Fluid Administration
Adequate administration of fluids is the first fundamental step in reducing the risk for contrast-induced nephropathy and intraprocedural hypotension in preparing patients for CAS.
Anticoagulation
As in most vascular procedures, intraprocedural anticoagulation is necessary with CAS. Adequate anticoagulation is usually achieved with unfractionated heparin (70 to 100 units/kg) administered after arterial access is gained and before manipulation of catheters in the aortic arch. An activated clotting time not longer than 250 to 300 seconds is recommended to avoid the risk for intracerebral hemorrhage from reperfusion. Heparin is rarely reversed at the end of the procedure. Given that the half-life of heparin is about 90 minutes, the procedure is usually finished before the anticoagulant effect of the initial dose has completely worn off. Bivalirudin may be used as an alternative.124
Atropine
Hypotension associated with marked bradycardia is a common feature after balloon dilatation, especially in elderly patients with heavily calcified stenoses.150–152 Intravenous atropine (0.4 to 1 mg given intravenously) may be administrated before stent deployment and balloon inflation to suppress the hemodynamic response to stretching of carotid baroreceptors. In the case of a severe hemodynamic response, aggressive volume expansion and an additional dose of atropine or use of vasopressors, including intravenous phenylephrine (1 to 10 µg/kg/min) and dopamine (5 to 15 µg/kg/min) infusion, may be necessary.124 Moderate hypotension may last 24 to 48 hours before the carotid sinus adapts to the radial force of the self-expanding stents, and pharmacologic support is occasionally required. The hemodynamic response may be exaggerated in patients with baseline bradycardia or those taking beta blockers or digoxin, whereas patients with a denervated carotid sinus because of previous CEA or with pacemakers are at lower risk. Intraprocedural transcutaneous temporary cardiac pacing is rarely indicated for CAS.153,154
Vasodilators and Hypotensive Drugs
Accurate pressure control during and after the procedure is essential in decreasing the risk of complications. Hypertension occasionally develops immediately before, during, or rarely after CAS, and maintenance of systolic blood pressure below 180 mm Hg is advised to minimize the risk of intracranial hemorrhage.124 More commonly, hypotension may develop after stent deployment and persist a few days as described before.
Spasm may appear after EPD retrieval in the distal ICA. Most cases of spasm resolve spontaneously in a few minutes. However, when spasm is severe and persistent, administration of vasodilators may be required. Commonly, nitroglycerin (100 µg) is administered directly into the ICA through the introducer sheath or guiding catheter (500 µg diluted in 10 mL and 2 mL). Additional doses may be administered every 3 to 5 minutes. The patient must be observed for possible exaggerated hypotension.
Periprocedural Therapy
Several developments in medical treatment in preparation for or after CAS have evolved since the 1990s, including newer antiplatelet agents, statins, and the recognized benefits of lowering blood pressure.123–125,127–129
Antiplatelet Drugs
Rapid thrombus formation inside the stent and consequent potential embolization immediately after CAS provide the rationale for double antiplatelet therapy.123–125,127–129,155 Aspirin, the most studied antiplatelet agent, causes irreversible inhibition of platelet cyclooxygenase by decreasing production of thromboxane A2.155–158 However, there is interpatient variability in the response of platelets to aspirin as well as occasional resistance to the drug.159 Thienopyridine derivatives, such as ticlopidine and clopidogrel, or the newer ticagrelor and prasugrel, which act as adenosine diphosphate receptor antagonists through a separate pathway from inhibition of cyclooxygenase, have been considered a valid alternative or adjunctive treatment to aspirin.160–162
Clinical evidence supporting the use of double antiplatelet treatment to prevent vascular events is mainly derived from studies involving coronary interventions; there are few data in CAS patients.163–170 The benefit is less clear when dual treatment is applied to patients who had ischemic stroke or transient ischemic attack.171,172 The MATCH trial (Management of Athero Thrombosis with Clopidogrel in High-risk patients with recent transient ischemic attack or ischemic stroke) found only a nonsignificant 6.4% reduction in the relative risk for primary endpoints (stroke, MI, vascular death, rehospitalization) with a dual antiplatelet regimen.172 Similar results were obtained in the CHARISMA trial (Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management and Avoidance).173,174 The benefit of combination therapy became marginally significant in high-risk subgroups, such as patients with symptomatic vascular disease; conversely, the related risk of bleeding obviated the benefits of treatment in low-risk subgroups.173,174
Despite uncertain information about the effectiveness of dual antiplatelet treatment in preventing cerebrovascular ischemic events during CAS, there is consensus that patients undergoing CAS should receive a regimen similar to that of those undergoing coronary stenting, as recommended by guidelines from the Society for Vascular Surgery, American College of Cardiology, American Heart Association, European Society of Cardiology, and European Society for Vascular Surgery.123–125,127 The standard regimen includes aspirin, 75 to 325 mg/daily, and clopidogrel, 75 mg/daily, both starting at least 4 days before the procedure. Alternatively, a loading single dose of clopidogrel (300 to 600 mg) at least 4 to 6 hours before the procedure is advocated. For patients intolerant to clopidogrel, ticlopidine (250 mg twice daily) may be used.123
There is limited evidence to support the benefit of dual antiplatelet therapy with aspirin and dipyridamole after CAS.128,175,176
Today, most centers administer dual antiplatelet therapy for 4 weeks after carotid stenting as suggested by studies documenting the occurrence of adverse events during the first 24 days and up to 30 days after the procedure and taking into account that stent endothelialization lasts 28 to 96 days.124,127,177 Longer dual antiplatelet treatment is suggested in patients at high risk for restenosis or major cardiovascular events.127,163,165,168 A low dose of aspirin (75-100 mg/daily) may be considered effective for long-term treatment in standard-risk patients.
Regarding the patients scheduled for combined carotid and coronary surgery, the American College of Cardiology and American Heart Association guidelines recommend stopping clopidogrel therapy at least 5 days before CABG.123–125,127,178 Recent data suggest that clopidogrel may be safely continued through the perioperative period without excessive bleeding risk, and the last Society for Vascular Surgery carotid guidelines suggest that it is reasonable to individualize perioperative management of clopidogrel therapy.123
Antithrombotic Drugs
Few studies have compared antiplatelet with anticoagulant therapy after CAS. In a small RCT that included 47 patients undergoing CAS, the 30-day neurologic complications rate was 0% with clopidogrel and aspirin versus 25% (P = .02) with aspirin plus heparin. The unacceptable level of complications in the anticoagulant group resulted in early termination of the study.179
Warfarin is recommended for primary and secondary prevention of stroke in patients with atrial fibrillation.180,181 On the basis of these trials in patients with carotid stenosis and a concurrent risk for cardioembolic stroke, it is reasonable to maintain the antithrombotic therapy without adjunctive antiplatelet drugs to avoid the increased hemorrhagic risk. Warfarin may be converted to intravenous heparin before the procedure.
Statins
Statins (3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors) have been found to be highly effective in preventing stroke in both male and female patients with cardiovascular disease.182 Review and large multicenter studies analyzing the effect of statins in patients undergoing vascular surgery showed significant improvement in postoperative outcomes.183–185 A number of studies have specifically focused on outcomes of carotid surgery, raising the question of whether similar effects would affect the outcomes of CAS, but few studies have addressed this issue.185–189 The supposed benefit in patients undergoing carotid revascularization appears to be largely independent of the cholesterol-lowering effect of the drug; it is due to so-called pleiotropic effects because statins have been shown to stabilize atherosclerotic plaque and show anti-inflammatory, antithrombogenic, antiproliferative, and antileukocyte adhesion properties.
In a single-center experience, the incidence of cardiovascular events after CAS was 4% in statin users versus 15% in nonusers (P < .05).188 Another large series showed 1.5% versus 4% 30-day stroke/death (P < .009) for users and nonusers.189 On the basis of available data, it is reasonable to prescribe statins early before the procedure but also to monitor liver function and creatine kinase levels thereafter to detect potential side effects of the drug.124,127
Procedure Technique
The CAS procedure widens the carotid stenosis by plaque dilatation and attempts to prevent future embolization by scaffolding the ruptured plaque against the vessel wall. The specific technique of CAS may vary according to operator experience. However, some steps are widely accepted in standardized protocols.
Technologic Requirements
In the optimal setting, carotid intervention should be performed in angiography suites specifically equipped with a sterile environment and all facilities necessary for an operating room, as in the so-called hybrid operating rooms.23,152,178,190 In general, specific minimum requirements are the following: appropriate equipment and sufficient space to allow positioning of patient monitoring and anesthesia equipment while preserving the sterile field; high-resolution image intensifier with the ability to acquire and to store high-quality images with the lowest radiation exposure; and adequate physiologic monitoring system and prompt access to surgical and medical emergency support.
It has been suggested that a bank of three monitors be used to show simultaneous working and reference images as well as hemodynamic data. The monitors should be positioned in front of the operator to allow thorough understanding of the patient’s condition and progress of the procedure. Prompt availability of a large inventory of endovascular supplies as well as any emergency equipment and medication is critical for a successful carotid stent program.
Periprocedural Monitoring
One important aspect of performing CAS safely is ensuring that the patient is adequately monitored before, during, and after the procedure. Intraprocedural management includes continuous assessment of neurologic and hemodynamic parameters and anticoagulation. Continuous electrocardiographic, pulse oximetry, and intra-arterial pressure monitoring through the side arm of the introducer sheath should be routinely used during the procedure to detect early and to treat hemodynamic alterations.
The level of alertness, speech, and motor function must be continuously evaluated by asking the patient to answer simple questions and to squeeze a plastic toy in the contralateral hand. However, because subtle ischemic neurologic changes may be overlooked, more accurate assessment is advisable after completion of the procedure. Transcranial Doppler (TCD) may be used as an adjunctive technique to monitor the patient during and immediately after the procedure. Indeed, it is known that cerebral embolic events may occur during any phase of CAS. High-intensity transient signals can be detected as warning signs.191 Because reflection of ultrasound power depends on both the size and composition of the embolus, the use of multifrequency TCD has been suggested for automatic recognition of high-intensity transient signals and differentiation between particulate emboli and air bubbles or artifacts.192,193 Nevertheless, sources of error are frequent, and the ability of TCD to differentiate emboli with relevant clinical consequences from artifacts is questionable.141,142,194–197
Mild sedation may be offered to CAS patients, but an adequate level of consciousness under local anesthesia should be maintained to monitor the neurologic condition. Noninvasive hemodynamic monitoring should be maintained until the following morning. Postprocedural management for 24 hours should include ultrasound evaluation to assess the morphology of the treated vessel and the arterial access site. Laboratory chemistry including cardiac enzymes to rule out renal and cardiac damage should be performed before discharge and after 1 week.
At discharge, the patient should be instructed to comply with the follow-up schedule and to inform the practitioner in the event of any new neurologic symptoms. Serial follow-up assessment by trial convention should be performed at 1 month, 6 months, and annually thereafter and should include duplex ultrasound imaging to detect restenosis.124
Procedure
Access
Transfemoral.
Retrograde right femoral access followed by a 6F to 9F introducer access sheath is the most commonly used technique for catheter manipulation by a right-handed operator. The left common femoral is the second choice if the right femoral route is not available. Insertion of guide wires and catheters should follow the method for any endovascular procedure. Full-dose heparin is administered intravenously at this step.
Transcervical.
A direct CCA access at the base of the neck has recently been introduced and can be used in selected cases.198–202 The rationale of the transcervical CCA access approach is to avoid catheter manipulation in a diseased aortic arch, which can increase the risk of plaque embolization into the brain.198–202 A percutaneous approach with puncture of the CCA at the base of the neck with ultrasound guidance can be used to obtain a stable platform to next advance the EPD and stent to the proper carotid site. A direct percutaneous CCA approach may be complicated with dissection or embolization in vessels with diffuse atherosclerotic lesions. In such situations, a surgical cutdown with a 2- to 3-cm neck incision and surgical exposure of the CCA, even if it is more invasive, might be safer. In all cases of transcervical approach, a careful evaluation of the level of carotid bifurcation is needed because a short CCA may not allow a stable placement of a short introducer sheath.
Transbrachial and Transradial.
A transbrachial access may be employed with a 6F guiding catheter in case of aortoiliac occlusion or extremely difficult access to the CCA, as in a type III arch with diffuse calcification. A transradial approach has recently been suggested as an alternative with the same indications.203
Remote Access to the Target Lesion
Stable sheath access in the proximal CCA is a critical step. Vessels that originate below the apex of the aortic arch are more difficult to cannulate because wires and catheter insertion and exchange become increasingly difficult with a type II or III aortic arch.
In procedures with remote access, two main techniques to advance the catheter into the CCA are commonly used. Once it is in place, the guiding catheter or long sheath side port is intermittently irrigated or attached to a slow continuous infusion of saline to avoid stagnation of blood in the sheath and then connected to a blood pressure monitoring system.
Sheath Based.
In the sheath-based platform, preshaped 5F catheters, such as the Judkins 4, Headhunter, Simmons 1, or other models, may be used for CCA cannulation according to the preference of the operator. Through these catheters, an exchange-length stiff 0.035-inch guide wire is placed in the terminal branches of the external carotid artery (ECA), and a 6F 90-cm sheath (e.g., Shuttle, Cook, Inc.; Destination, Terumo Interventional Systems) with its dilator is then advanced over the wire and positioned with a pull-and-push maneuver in the distal CCA a few centimeters below the bifurcation. Care must be taken to identify the tip of the dilator because it should be kept away from the carotid bulb. Otherwise, a 125-cm curved catheter premounted into the sheath is used for direct cannulation and advancement of the guide wire and sheath (telescopic technique). The telescopic method is preferable in patients with a calcified and tortuous aortic arch because the catheter and guide wire assembly provides additional support with fewer maneuvers. One possible disadvantage is the mismatch between the size of the catheter (5F) and the sheath, which may cause the tip of the sheath to scrape the arterial wall (“snowplowing”) and result in distal embolization.
Guiding Catheter Based.
As an increasingly used alternative, CCA access is achieved directly with a preshaped guiding catheter (e.g., multipurpose curve, vertebral or reversed-angle Vitek catheter) with a 6F to 7F diameter that is left at the CCA level for the entire procedure. The advantage of this technique is that it does not require exchange wires and catheter, with consequent reduced manipulation approaching the target lesion. A shortcoming of the approach is the need to reach a stable position well above the CCA origin to avoid displacement of the guiding catheter into the aortic arch during stent progression. When the guiding catheter is judged not to be sufficiently stable in the CCA, a high-support 0.014-inch coronary “buddy wire” placed into the ECA may be used to better stabilize the working platform.
Arteriography
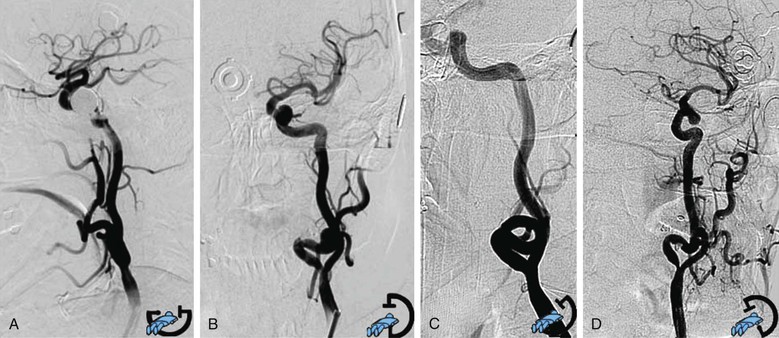
When a stable working platform has been reached in the CCA, an arteriogram through the guiding catheter or sheath should be obtained. Anteroposterior or lateral projection is needed to obtain minimal overlap of the ICA and ECA, optimal visualization of the target lesion, and complete intracranial carotid vessel distribution. When differentiation between the two carotid branches is not easily obtained with two projections, an adjunctive oblique projection is performed following the best view obtained from the preprocedural three-dimensional computed tomographic angiography reconstruction. Rotational angiography (the image intensifier rotates around the patient and acquires a series of images that are then reconstructed through software algorithms into a three-dimensional image) is occasionally used, when it is available, in selected cases of difficult visualization of the carotid lesion (Fig. 101-3).
Stent System Delivery
Embolic Protection Device Deployment.
After full angiographic evaluation, a decision must be made about use of an EPD. Several EPDs are available, and selection depends on lesion characteristics and anatomic considerations. With the most commonly used distal filter, the stenosis is crossed with a 0.014-inch guide wire that is a fixed or mobile component of the EPD in use, usually with a road mapping technique. Distal filters are deployed into the ICA, before its petrous segment; the position must be checked throughout the entire procedure. It is important not to advance the tip of the device any farther because the intracranial portion of the carotid artery is highly prone to dissection and spasm. Different types of EPDs are presented later in this chapter.
Predilatation.
When the stenosis is extremely severe, after placement of the EPD, predilatation with a 2.5- or 4.0-mm coronary balloon at relatively low inflation pressure (4 to 6 atm) may prevent difficulty in stent crossing and release. Rarely, careful predilatation may be required to allow EPD deployment through a preocclusive stenosis. In these settings, use of a flow reversal EPD is an alternative (see later section on selection of EPD type for details).
Stent Delivery.
The stent is deployed under road mapping control or by use of the vertebral bones as landmarks. The stent is usually placed across the bifurcation because most of the time the plaque extends to the bulb area. A self-expanding stent is used; a variety of diameters (7 to 10 mm), lengths (3 to 4 cm), and shapes (cylindrical or tapered) are available. The diameter of the stent must be sized to the largest portion of the vessel, typically the distal CCA. Different models of stents can be used to better adapt to the vessel morphology and plaque characteristics. This issue is discussed later in the chapter.
Postdilatation.
After stent deployment, short (2 cm), usually 5- to 5.5-mm balloons are used to dilate the narrowest portion of the stent. Balloon diameter should never exceed the diameter of the distal ICA. The balloon is always maintained within the stent. Higher pressure might be needed for heavily calcified plaque, which has a tendency to recoil.
Retrieval of the Embolic Protection Device and Completion Angiography.
A completion arteriogram of the carotid bifurcation at highest magnification is obtained before removal of the protection device to ensure the accuracy of deployment and to evaluate possible residual stenosis, spasm at the stent endpoint, or filling defects inside the stent due to plaque protrusion. After retrieval of the guide wire and EPD, a final completion angiogram of the cervical carotid and intracranial circulation is obtained, usually in double projection. ICA spasm may occur distally from filter movements during guide wire manipulations. Typically, watchful waiting and occasionally the administration of small doses of nitroglycerin (100-200 µg) through the guiding sheath allow resolution of the problem.
Care should be taken in the total amount of contrast agent used because of potential toxicity for the brain parenchyma. The total amount injected is optimally maintained as low as possible and never exceeding 60 mL.
Access Hemostasis.
Heparin’s action is not usually reversed. Access site hemostasis may be achieved with a percutaneous closure device or groin manual compression.
Embolic Protection Devices
EPD use is suggested to reduce the occurrence of distal cerebral embolization and, potentially, the risk of stroke. However, debate concerning the routine use of EPDs during CAS is still open because of different results reported. The EVA-3S trial provided strong support for the use of EPDs: a 30-day risk of stroke of 7.9% with protected CAS versus 25% with unprotected CAS.17 On the other hand, the SPACE trial found the same rate of events in both groups; the risk of 30-day death or any ipsilateral stroke was 6.2% in the protected group and 8.3% in the unprotected group (P = .40).204 In the CREST and in the postmarketing CAPTURE 2 registry, the use of an EPD was mandatory when feasible, and it was used in 96% of CREST patients. The systematic use of EPDs in CREST has been proposed as a main factor in the relatively low rate of adverse events in this RCT compared with others.28,62–64 In a meta-analysis by Garg et al205 that included a total of 12,263 protected and 11,198 unprotected CAS procedures, the relative risk for stroke was 0.62 (95% CI, 0.54-0.72) in favor of protected CAS. The pooled stroke rate at 30 days was 2.6% with use of an EPD versus 4.2% in patients without protection (P < .001).
Use of an EPD reduced the risk of stroke during CAS by 38% in all patients (33% in the symptomatic and 39% in the asymptomatic patients; P = .05).205 However, conflicting comments have been raised about the specific risk for stroke from improper placement of the EPD, and some investigators argue for selective use.88,94,206–210
Currently, use of an EPD is required by the Centers for Medicare and Medicaid Services (CMS) to qualify for reimbursement. The updated Society for Vascular Surgery guidelines for management of carotid disease of 2011 released a grade I, level B recommendation for the use of an EPD during CAS to reduce the risk of cerebral embolization.123
Categories of Embolic Protection Devices
Today there are a large variety of EPD models with different mechanisms that extend its applicability to disparate anatomic conditions. Three conceptually different methods, each with advantages and drawbacks, have been developed.
Distal Filter.
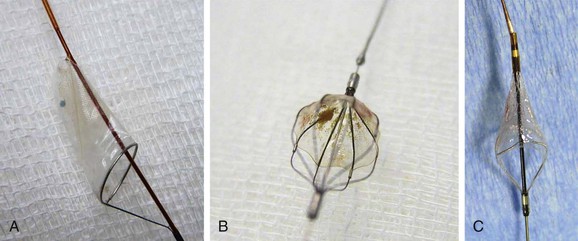
Filter-type EPDs, the most widely used devices,77,104,208–211 allow antegrade cerebral flow during the entire procedure. Filter design varies, with no demonstrated superiority of one model over another212; some are advanced over a 0.014-inch wire, and others are attached to the guide wire with a steerable wire tip. Crossing profiles usually range from 1.7F to 3.9F. Distal filter systems function like an umbrella or basket that is opened in the ICA and placed in the distal ICA after the lesion is crossed to capture any debris during the procedure (Fig. 101-4). After successful angioplasty and stent placement, the distal filter containing the debris is removed with a dedicated retrieval system. The main advantages of these EPDs are the maintenance of blood flow to the brain, the availability of angiographic control throughout the procedure, and the reduced need of device manipulation inside the carotid vessels in proximity of the target lesion. The main drawbacks are the need to cross the ICA lesion before delivery of the EPD, the difficulty in crossing very tight and tortuous lesions, and the uncertainty of capturing all debris for incomplete arterial wall apposition.
Distal Occlusion Balloon.
Distal occlusion balloons are available but have generally been abandoned.8,93 Protection is obtained by occluding the distal ICA above the lesion with an inflated balloon and flushing and suctioning after stent deployment. Their main disadvantages include the need to cross the lesion without protection, as with filters; the potential inability to remove all of the embolic material; and the risk for distal spasm or wall damage as a result of balloon inflation in the distal ICA.
Proximal Protection Devices with Flow Stasis or Reversal.
Proximal protection systems allow embolic protection before the lesion is crossed by use of flow stasis or flow reversal. The Mo.Ma system uses two occlusion balloons, one each in the CCA and the ECA, in a single delivery catheter. Debris is recruited with intermittent syringe aspiration through the long introducer sheath, after stent deployment and balloon dilatation but before deflation of the occlusion balloon.52
The Gore Flow Reversal System is based on the same principle of ECA and CCA balloon occlusion, but an external shunt, filtering all the debris, is placed between the long introducer sheath in the carotid artery and a previously placed short introducer in the femoral vein.57 Similarly, the most recent MICHI Neuroprotection System allows a reverse-flow arteriovenous circuit to be obtained by connecting an 8F transcervical arterial access sheath and an 8F venous return sheath with a large blood flow line with flow controller.201
The main advantage of these new EPDs is that no interaction with the plaque occurs until the stasis or reversal of flow is initiated. The main disadvantages are the increased need of device manipulation in areas close to the target lesion; the relatively large size of the access sheath (9F-8F for femoral approaches, 8F for transcervical approach); and the need to occlude both CCA and ECA, causing flow stasis or reversal, with increased possibility of neurologic intolerance. This condition is generally encountered in about 5% to 9% of patients and can be managed by short intermittent occlusion times.123,201,211
Selection of Embolic Protection Device Type
The selection of filter device versus proximal or flow reversal systems is guided by the patient’s anatomy but often related to operator preference. It is hoped that technologic improvements including lower profile of the device, better efficacy in recruiting debris, and reduced need of manipulation for the proximal occlusion balloon systems can improve the safety of the procedure.*
A number of studies, mostly industry sponsored, have been conducted to show the safety of different EPDs.203,213,214 Randomized (e.g., PROFI trial)214 and mainly not randomized studies, including small samples of patients, on direct comparison between proximal occlusion and distal filter, seem to show lower rates of periprocedural stroke213,214 or reduced rates of microembolic lesions with the proximal occlusion systems,198,201,202,215 but this awaits confirmation in larger population studies.
Despite considerable improvements, no EPD model has been shown to be completely effective, and the problem of cerebral emboli during CAS in different steps of the procedure (cannulation, placement of the EPD, balloon dilatation, and stenting) cannot be considered completely solved. The operator’s skill is crucial, and each interventionalist should be familiar with at least two different classes of EPD so that the technique can be tailored to the individual case.178
Stent Selection
Different self-expandable carotid stent models composed of either stainless steel (a cobalt alloy) or nitinol (a nickel-titanium alloy) can be applied to cover the entire carotid lesion. Flexibility (the ability to conform to vessel tortuosity) and scaffolding (the amount of support given to cover the plaque) are the two main characteristics that drive stent choice. In the case of tortuous anatomy, a rigid stent may create a kink at the distal end of deployment. Insufficient scaffolding may cause protrusion of plaque material through the stent strut and produce distal embolization. In general, nitinol stents have higher radial force, which is useful in counteracting recoiling of severely calcified plaque, but no one type of self-expanding stent has been shown to be superior to another.
Closed Cell versus Open Cell Stents
An important technologic feature of stents for the carotid artery has been the development of two main configurations: closed cell, in which all stent struts are interconnected; and open cell, in which not all struts are interrelated. Open cell stents are more conformable than closed cell stents and preferred in tortuous anatomy (Figs. 101-5 and 101-6).
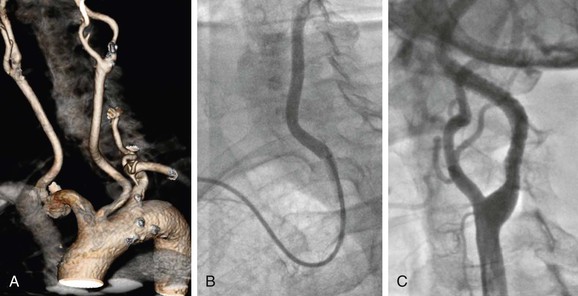
Figure 101-5 Difficult access procedure. A, Bovine aortic arch with left carotid stenosis. B, Right brachial access with 6F guiding catheter for difficult target vessel approach. C, Open cell stent has been placed for better conformability.
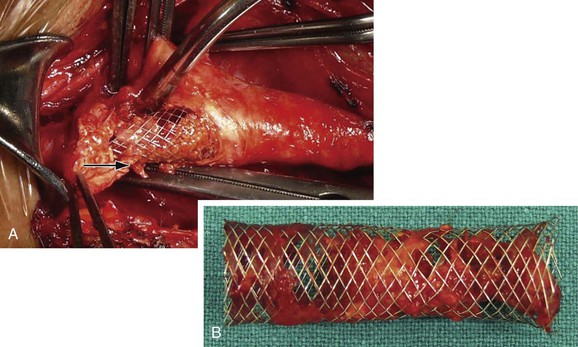
Figure 101-6 Postprocedural conversion to open surgery because of a transient ischemic attack. A, Plaque can be seen prolapsing through the stent strut (arrow). B, Carotid stent after removal with trapped plaque.
Stent selection is often based on the physician’s preference. Although it has been suggested that closed cell stents with a smaller free cell area and a greater percentage of wall coverage offer better scaffolding, reducing embolization risk compared with the open cell structure, there is no consensus on that point.72
In a multicenter experience of 3179 CAS procedures, Bosiers et al72 reported a significantly lower 1.3% adverse event rate in patients with closed cell stents and 3.4% in those with open cell stents (P < .0001), mainly for symptomatic patients. At the opposite, in another multicentric study of 1648 patients, Schillinger et al73 reported a similar 30-day stroke and death rate for closed cell and open cell stents (3.1% vs. 2.4%; P = 0.38). A small randomized study of open versus closed cell stents by Timaran et al216 failed to show increased stroke/death rate or new cerebral lesion by magnetic resonance angiography (53% vs. 47%) or cerebral embolic Doppler signals (264 vs. 339) in procedures performed with the two different types of stents. Histologic analysis of material retrieved within the distal filter suggested that the use of open cell stents was associated with a larger mean particle size but had no impact on procedural outcomes or total number of embolic particles compared with closed cell.217 More data are needed for final conclusions to be drawn about open versus closed cell stent selection or other characteristics that may ultimately determine optimal stent performance.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree