Fig. 1 a, b.
Coarctation of the aorta in an 11-year-old male who presented with upper extremity hypertension. a Axial MRI shows a normal sized ascending aorta (AA) and a markedly small descending aorta (arrow) in the region of the aortic coarctation. PA, pulmonary artery. b 3D volume rendered image demonstrates marked focal narrowing (arrow) of the descending aorta, consistent with aortic coarctation. Enlarged collateral vessels, including those of the internal mammary artery and intercostal arteries, are also seen
In neonates with coarctation of the aorta, increased pulmonary vascularity with cardiomegaly is often seen on chest radiographs. In older children with delayed diagnosis of aortic coarctation, the “figure of 3” sign, which reflects prestenotic dilatation of the aortic arch and left subclavian artery, indentation at the coarctation site, and post-stenotic dilatation of the descending aorta, may be present [5–7]. Other typical concomitant findings are collateral vessel formations, including those of the internal mammary, intercostal, and superior epigastric arteries (Fig. 1b). In particular, enlarged intercostal arteries can result in the development of ribnotching under the posterolateral aspects of the fourth to eighth ribs [5–7]. Additionally, the bicuspid valve can be seen in 25–50% of patients with aortic coarctation [5–7]. In the evaluation of coarctation of the aorta using cross-sectional imaging studies such as multi-detector CT, multiplanar (2D) and 3D images performed better than axial CT images alone [8].
Three currently used management options for aortic coarctation include surgical resection of the narrowed segment followed by primary end-to-end anastomosis, balloon angioplasty, and placement of a vascular stent. Imaging evaluation also plays an important role in the evaluation of residual or recurrent aortic coarctation as well as complications such as aortic dissection or pseudoaneurysm formation [5–7].
Cardiac Septal Defect
Cardiac septal defect refers to a hole or discontinuation in either the atrial or the ventricular septum, or both. Affected pediatric patients with small septal defects come to attention because of murmur on physical examination. By contrast, affected pediatric patients with moderate to large septal defects typically present early in life (1–3 months) with congestive heart failure due to pulmonary arterial overcirculation, which results from left-to-right shunt via the septal defect.
Ventricular Septal Defect
Ventricular septal defect (VSD) is the second most common congenital cardiac malformation, accounting for approximately 20% of all congenital cardiac anomalies [1–3]. On physical examination, systolic murmur is present because blood flow across the VSD is primarily during systole. Based on the location of the defect, VSD is classified into four different types: (1) subpulmonary type, caused by a deficiency of the conal septum; (2) membranous type, in which the defect is located posterior to the septal leaflet of the tricuspid valve; (3) atrioventricular canal, which is caused by a deficiency of the endocardial cushion component of the interventricular septum; and (4) muscular, which is the most common type of VSD and can occur in any portion of the muscular septum.
Although chest radiographs may be normal in affected pediatric patients with small VSDs, increased pulmonary vascularity due to an underlying left-to-right shunt (i.e., shunt vascularity), an enlarged main pulmonary artery, biventricular enlargement, and left atrial enlargement are present on chest radiographs in pediatric patients with moderate to large VSDs (Fig. 2). Shunt vascularity refers to an increase in the number and caliber of pulmonary vessels resulting from the increased arterial flow. CT and MRI can directly show the discontinuation of the ventricular septum in affected patients.
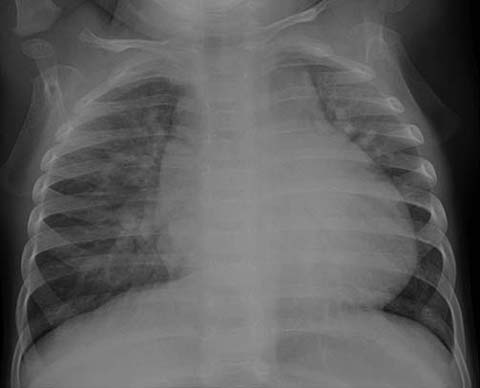
Fig. 2.
Ventricular septal defect in a 13-month-old male. Chest ra-diograph shows increased pulmonary vascularity and cardiomegaly
While small VSDs (also called restrictive VSDs) often close spontaneously or can be managed with a percutaneous septal occlusion device, hemodynamically significant moderate to large VSD are usually treated surgically with a patch graft. Infectious endocarditis is a potential complication of untreated VSD.
Atrial Septal Defect
Atrial septal defect (ASD) is the most common shunt lesion detected in adults, accounting for approximately 10% of congenital heart anomalies [1–3].
On physical examination, diastolic murmur is present because blood flow across the VSD is primarily during diastole. Based on the location of the defect, there are three subtypes of ASD: (1) ostium primum, in which the defect is located in the anteroinferior portion of the septum; (2) ostium secundum, in which it is located in the midportion of the atrial septum; and (3) sinus venous defect, in which the defect is located at the superior portion of the interatrial septum, near the superior vena cava (SVC).
Although chest radiographs can be normal in affected pediatric patients with small ASDs, increased pulmonary vascularity due to underlying left-to-right shunt, an enlarged main pulmonary artery, right heart enlargement, and a normal size left atrium and aorta are typically seen in patients with moderate to large ASDs (Fig. 3a). CT and MRI can directly show the exact location and extent of an ASD as well as associated anomalies, such as anomalous drainage of the right superior pulmonary vein, which is highly associated with ASD of the sinus venous defect type (Fig. 3b).
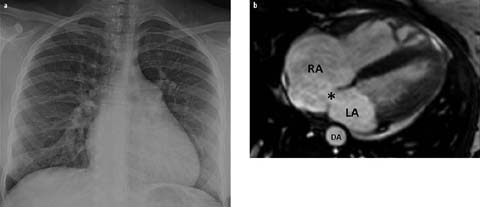
Fig. 3 a, b.
Atrial septal defect in a 14-year-old female. a Chest radiograph shows increased pulmonary vascularity, a dilated main pulmonary artery, and cardiomegaly. b Axial MRI demonstrates an atrial septal defect (asterisk) along with enlargement of the right atrium (RA). LA, left atrium; DA, descending aorta
Small ASDs often close on their own during infancy or early childhood or they can be managed with a septal occlusion device [9]. Surgical repair is typically necessary for managing large or multiple ASDs.
Transposition of the Great Arteries
TGA refers to a congenital malformation of the great arteries in which they are reversed in their origins from the heart (Fig. 4). There are two different types of TGA: D-transposition of the great arteries (D-TGA) and L-transposition of the great arteries (L-TGA) [1–3]. In D-TGA, the aorta arises from the morphologic systemic right ventricle and the pulmonary artery arises from the morphologic systemic right ventricle, resulting in ventricular arterial discordance. The pulmonary and systemic circulations exist in parallel, requiring bidirectional shunting between the two sides of the heart via an ASD, VSD, patent ductus arteriosus (PDA), or patent foramen ovale for survival. By contrast, congenitally corrected TGA, or L-TGA, is characterized by both ventriculoarterial and atrioventricular discordance. The pulmonary and systemic circulations are in series rather than in parallel as in D-TGA. Systemic venous return flows to the right atrium, enters the morphologic left ventricle, and is eventually delivered to the pulmonary circulation via the main pulmonary artery. Pulmonary venous return subsequently flows to the left atrium, enters the morphologic right ventricle, and is delivered to the systemic circulation via the aorta. While patients with D-TGA usually present with cyanosis during the first day of life, those with L-TGA are typically asymptomatic.
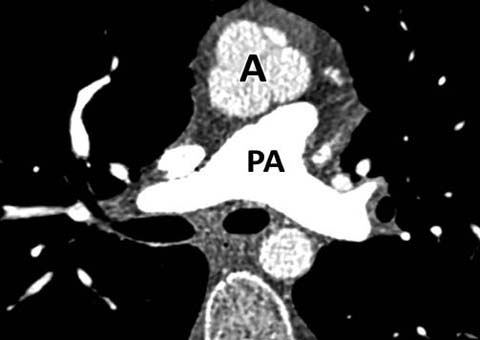
Fig. 4.
D-transposition of the great vessels (D-TGA). Axial CT shows the aorta (A) located anterior to the pulmonary artery (PA)
On chest radiographs, increased pulmonary vascularity and mild to moderate cardiomegaly are usually seen in patients with D-TGA [1–3]. Additionally, the superior mediastinum is narrowed because of the abnormal position of the great vessels and a concave main pulmonary artery. The constellation of these findings results in the radiographic appearance of the cardiomediastinal silhouette, referred to as the “egg-on-a-string sign” [2]. CTA or MRA is useful for the assessment of postoperative complications including narrowing of the anastomosis of the aorta or pulmonary artery in addition to branch pulmonary artery stenosis and coronary artery narrowing.
Corrective surgery via a Jatene “arterial switch” procedure is required for managing patients with D-TGA in the first few days of life [1–3] (Fig. 5). By contrast, patients with congenitally corrected L-TGA may require pacemaker placement for progressive right ventricular dysfunction and conduction abnormalities in later life.
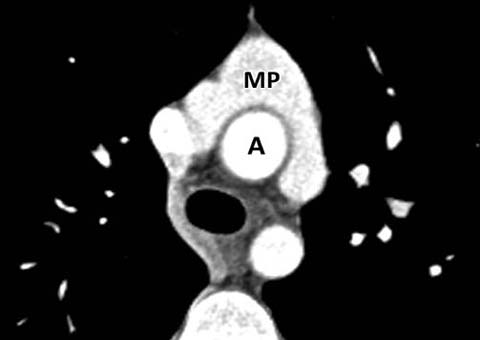
Fig. 5.
Jatene procedure. Axial CT after an “arterial switch proce-dure” for TGA shows characteristic draping of the main pulmonary artery (MP) around the aorta (A)
Ebstein’s Anomaly
Ebstein’s anomaly is the most common cause of marked cardiomegaly in the newborn period. It is characterized by the displacement of the septal and posterior leaflets of the tricuspid valve towards the apex of the right ventricle of the heart [10]. This results in “atrialization” of a portion of the morphologic right ventricle and subsequent enlargement of the right atrium and a reduction in the size of the anatomic right ventricle [10]. This condition may be an isolated finding or associated with other congenital heart defects, such as a patent foramen ovale or an atrial septal defect, allow the passage of a right-to-left shunt. Although patients with mild Ebstein’s anomaly may be asymptomatic, in those with severer forms of the disease cyanosis and systolic murmur are often present [10].
On radiographs, decreased pulmonary vascularity, a concave pulmonary artery segment, and marked cardiomegaly due to underlying right atrial enlargement are characteristically seen [2, 10] (Fig. 6a). In affected patients with concomitant PDA, the pulmonary vascularity may be increased. CT and MRI can better demonstrate a small right ventricle and atrialized right ventricle (Fig. 6b), and MRI is better than echocardiography in visualizing the morphology of the anterior leaflet of the tricuspid valve and right ventricular free wall.
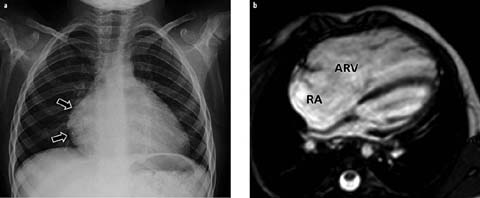
Fig. 6 a, b.
Ebstein’s anomaly in a 4-year-old female. a Chest radiograph shows decreased pulmonary vascularity and cardiomegaly with a prominent right heart (arrows). b Axial MRI demonstrates a large atrialized superior right ventricle (ARV). RA, right atrium
Tricuspid annulopasty and plication of the atrialized portion of the right ventricle are the current management procedures of choice for symptomatic pediatric patients with severe Ebstein’s anomaly.
Tetralogy of Fallot
Four anomalies of the cardiac structures comprise TOF: VSD, infundibular pulmonary stenosis, an overriding aorta, and right ventricular hypertrophy. It is the most common congenital heart defect resulting in cyanosis. A right aortic arch with a mirrorimage branching, peripheral pulmonary arterial stenosis, and an anomalous anterior descending coronary artery that courses over the right ventricular outflow tract are also often associated with TOF. Affected pediatric patients typically present with cyanosis in the first 6 months of life.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


