15 Cardiomyopathies
Dilated Cardiomyopathy
 Pathoanatomical and Pathophysiological Basics
Pathoanatomical and Pathophysiological Basics
Dilated cardiomyopathy (DCM) is characterized by dilatation of the heart chambers with reduction in systolic ventricular function. The left ventricle is most frequently affected, but in many cases all chambers are dilated, including both atria. The dilatation is associated with an increase in myocardial mass, due to hypertrophy of cardiomyocytes, and a marked increase in interstitial fibrosis.
Identification of the cause may not be possible in every case. Besides past or persistent myocarditis, other prominent causes are genetic mutations that involve proteins of the cytoskeleton. In addition, exogenous toxic and autoimmune processes may be responsible. Men are more frequently affected than women; the average age of manifestation is around 50 years.
 It is important to differentiate these primary, frequently familial cardiomyopathies from secondary cardiomyopathies with known causes, as well as dilated forms of coronary, valvular, or hypertensive heart disease.
It is important to differentiate these primary, frequently familial cardiomyopathies from secondary cardiomyopathies with known causes, as well as dilated forms of coronary, valvular, or hypertensive heart disease.
Specific Pathophysiology
When the systolic function of the left (and right) ventricle is impaired, dilatation, which is frequently severe, can help to maintain a normal stroke volume at rest with reduced fiber shortening. However, this requires an increased wall tension and thus increased heart work. In addition to causing activation of numerous cytokine and peptide systems, the increased wall tension is also responsible for the development of a secondary compensatory hypertrophy.
In addition to systolic dysfunction there can be diastolic dysfunction in the sense of impaired compliance of variable degree with correspondingly increased or still normal diastolic left ventricular pressure. Therefore, there is no direct association between ventricular diastolic pressure and the extent of ventricular dilatation or cardiac output.
With severe dilatation of the left and right ventricles, usually a functional mitral and tricuspid regurgitation can be detected.
Four hemodynamic stages of dilated cardiomyopathy are differentiated depending on cardiac output and left ventricular end-diastolic pressure at rest and under stress (Table 15.1).
 Indication for Cardiac Catheterization
Indication for Cardiac Catheterization
By definition a primary dilated cardiomyopathy can only be diagnosed after other potential causes of left ventricular dilatation and systolic dysfunction have been excluded. This is therefore the main indication for cardiac catheterization: to exclude or confirm a dilated form of coronary or valvular heart disease, and to initiate timely treatment of the underlying cause of the left ventricular dysfunction if possible.
Even though patient history and symptoms together with noninvasive cardiac findings enable diagnosis and classification of severity of the left ventricular dilatation and dysfunction in DCM, the findings are in many cases not sufficient for a reliable differentiation between a primary and a secondary cardiomyopathy. It is important that the patient is sufficiently compensated at the time of the examination and that the appropriate precautionary measures for cardiac catheterization in patients with impaired ventricular function are carefully heeded (Chapter 9).
 Goals
Goals
 Evaluation of systolic and diastolic ventricular function
Evaluation of systolic and diastolic ventricular function
 Measurement of left ventricular volumes
Measurement of left ventricular volumes
 Exclusion or confirmation of primary coronary or valvular heart disease
Exclusion or confirmation of primary coronary or valvular heart disease
 Detection and quantification of functional mitral regurgitation
Detection and quantification of functional mitral regurgitation
 Measurement of systemic and pulmonary vascular resistances
Measurement of systemic and pulmonary vascular resistances
 Endomyocardial biopsy if inflammatory etiology is suspected
Endomyocardial biopsy if inflammatory etiology is suspected
 Procedure
Procedure
 Arterial and venous puncture (4F–6F sheaths)
Arterial and venous puncture (4F–6F sheaths)
 Coronary angiography
Coronary angiography
 Catheterization of the left ventricle with the pigtail catheter
Catheterization of the left ventricle with the pigtail catheter
 Right heart catheterization with placement of the catheter in PCW position (balloon catheter)
Right heart catheterization with placement of the catheter in PCW position (balloon catheter)
 Simultaneous pressure recording PCW/LV
Simultaneous pressure recording PCW/LV
 Determination of cardiac output (according to Fick or thermodilution)
Determination of cardiac output (according to Fick or thermodilution)
 Ventriculography
Ventriculography
 Calibration and measurement of left ventricular volumes (sphere)
Calibration and measurement of left ventricular volumes (sphere)
 Right heart catheter pullback with pressure recording
Right heart catheter pullback with pressure recording
 Left heart catheter pullback with pressure recording
Left heart catheter pullback with pressure recording
 Special Characteristics
Special Characteristics
Depending on the hemodynamics in an individual case the operator has to decide whether ventriculography with the required volume load can be performed without risk or whether noninvasive assessment of ventricular function is sufficient.
Patients with DCM are at increased risk during cardiac catheterization. The duration of the examination should be kept short and contrast medium administration should be limited to what is absolutely required.
For these reasons it is acceptable not to do a simultaneous left/right heart catheterization but rather to obtain the relevant parameter in a separate right heart catheterization, in case this examination has not already preceded left heart catheterization.
 Findings on Cardiac Catheterization
Findings on Cardiac Catheterization
Ventriculography
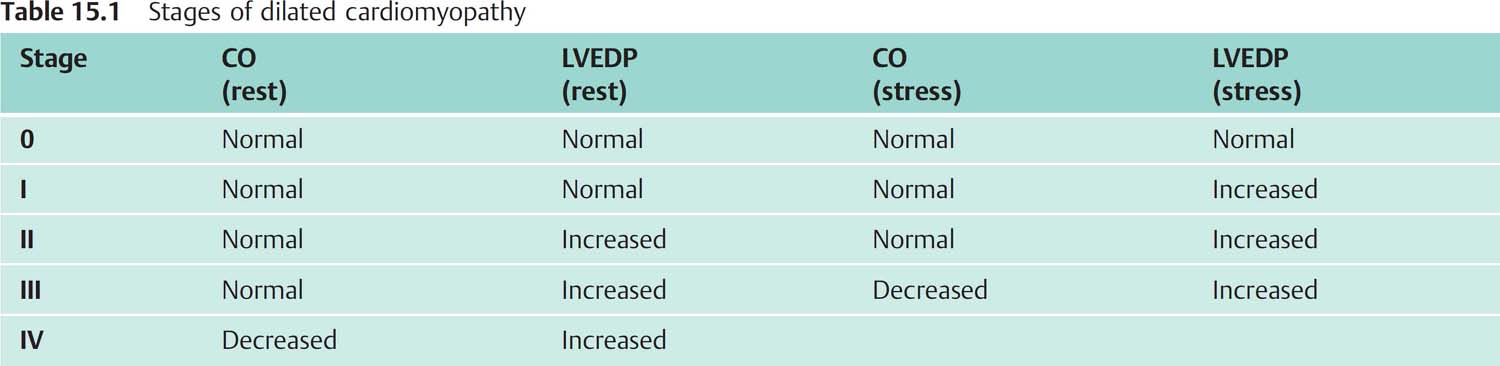
Characteristic features are a markedly dilated left ventricle in both systole and diastole with a rounded apex and globally impaired systolic function (Fig. 15.1a). The left ventricle has a spherical shape. Ejection fraction is impaired, the more so with increasing left ventricular dilatation. Of note, in DCM there is not infrequently a regional emphasis regarding the wall motion abnormalities. Potential causes of these regional impairments are myocarditis as well as thrombotic coronary emboli, derived from mural thrombi in the left ventricle.
Frequently, mild relative mitral regurgitation can be seen in DCM.
Pressures/Hemodynamics
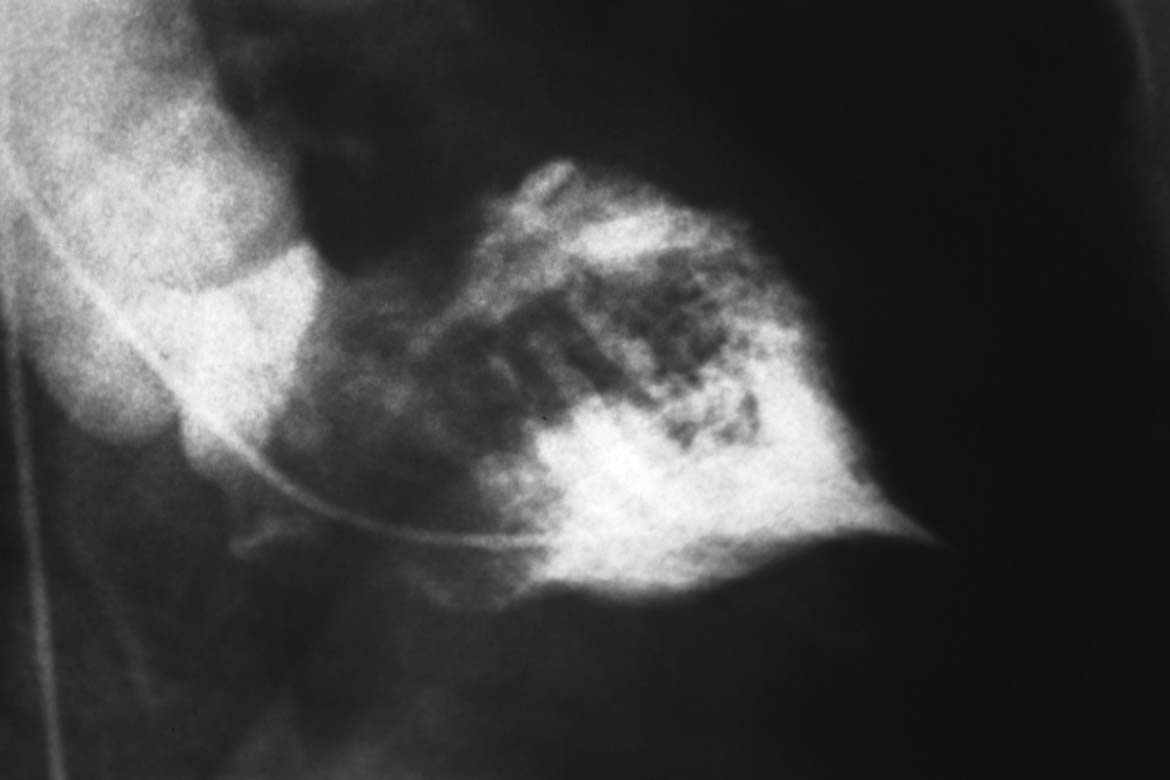
The marked systolic dysfunction of the left ventricle in patients with DCM leads to typical changes in left ventricular pressure with a triangular ventricular pressure tracing in systole. This is due to a slow upstroke, a short systole, and a slow decrease in systolic pressure (Fig. 15.1c).
Peak pressure in the left ventricle is usually reduced; values around 90 mm Hg are not uncommon. Left and right ventricular filling pressures are usually increased but they can also be in the normal range depending on compliance, patient symptoms, and treatment with diuretics and vasodilators. Depending on the stage of the DCM, cardiac output at rest can be either still normal or decreased (stage IV).
Systemic and pulmonary vascular resistances are usually increased. The extent also depends on the stage of the disease and on pretreatment with vasodilators.
Coronary Angiography
The coronary arteries appear as smooth, stretched and frequently large-caliber vessels without coronary stenoses. The epicardial coronaries thus convey an indirect indication of the extent of left ventricular dilatation. Otherwise there are no special features on coronary angiography.
 Interpretation of Findings and Patient Management
Interpretation of Findings and Patient Management
Left heart catheterization serves predominantly to ascertain the diagnosis DCM and to exclude a dilated form of coronary or valvular heart disease. All noninvasive methodologies also play a prominent role.
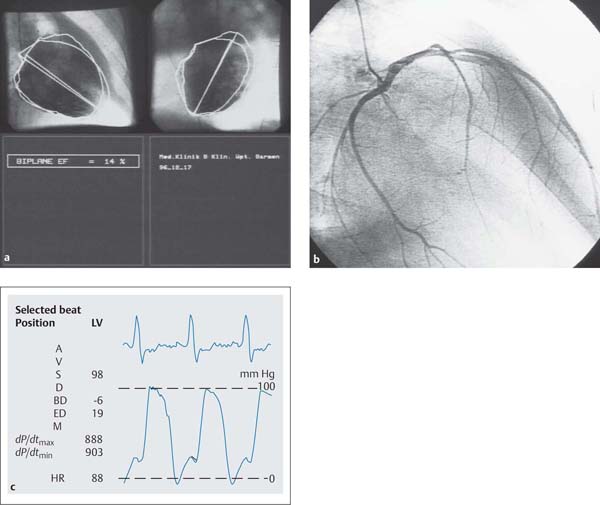
Fig. 15.1 a–c Dilated cardiomyopathy.
a Left ventriculogram with dilated LV, globally impaired contractility, spherical ventricular geometry, EF 14 %.
b Unobstructed, stretched left coronary artery (RAO projection).
c Ventricular pressure wave and hemodynamics.
Hemodynamics | |
Aorta: | > 108/68 mm Hg |
LVEDP: | 19 mm Hg |
PCW mean: | 14 mm Hg |
PA: | 29/12 (18) mm Hg |
RV: | 27/0–3mm Hg |
RA mean: | 2 mm Hg |
CO: | 3.8 L/min |
Cardiac index: | 2.17 (L/min)/m2 |
Pulmonary vascular resistance: | 84 dyn·s·cm−5 |
Systemic vascular resistance: | 1684 dyn·s·cm−5 |
Cardiac MRI can easily and precisely determine left ventricular mass, volume, wall motion abnormalities and ejection fraction. Late imaging after contrast medium administration (“delayed enhancement”) allows inferences regarding myocardial fibrosis and thus structure. Additional sequences to detect edema or a relative enhancement can indicate a possible inflammatory etiology.
Classification of severity and therapy are based on symptoms, left ventricular volumes and function, and hemodynamic results obtained during right heart catheterization. Additional findings, with some prognostic significance, can be gained from the molecular biological and immunohistological findings of endomyocardial biopsies and from the plasma levels or cardiac derived peptides (i.e., B-type natriuretic peptide).
Medical therapy consists of the administration of vasodilators and inhibitors of the renin–angiotensin–aldosterone system: ACE inhibitors/angiotensin receptor blockers, β-blockers, mineralocorticoid receptor antagonists, diuretics, and digoxin. In the case of electromechanical dyssynchrony (usually with left bundle branch block), there is the therapeutic option of cardiac resynchronization with implantation of a biventricular pacemaker with or without implantable cardioverter defibrillator (ICD) system (cardiac resynchronization therapy [CRT]).
In advanced stages of the disease the patient should be evaluated in a timely fashion for heart transplantation. The crucial observations for escalation of therapy are not findings on left heart catheterization but the persistence of severe symptoms (NYHA III–IV) when all medical options have been exhausted.
If a coronary artery disease is diagnosed as the cause of left ventricular dilatation and systolic dysfunction, further management depends on whether in functional tests (stress echocardiography, MRI, nuclear scan) the affected myocardial regions show signs of ischemia or viability. This is the only way to assess whether revascularization by PCI or CABG can be expected to improve clinical symptoms, ventricular function, and prognosis.
Hypertrophic Cardiomyopathy
 Pathoanatomical and Pathophysiological Basics
Pathoanatomical and Pathophysiological Basics
Hypertrophic cardiomyopathy (HCM) is characterized by pathological hypertrophy of the myocardium due to genetic mutations without apparent other cause. The exact relationship between inherited cause and sporadic forms is not precisely known.
Hypertrophic cardiomyopathy is in most cases a monogenetic disease with autosomal dominant inheritance, which affects both sexes equally. Currently more than 10 affected genes are known, which predominantly code for sarcomeric proteins. Mutations of the genes for β-myosin heavy chain, myosin-binding protein C, and troponin T account for ~70 to 80 % of cases.
Hypertrophic cardiomyopathy is characterized by the following structural and functional changes:
 Massive left ventricular hypertrophy in the following locations:
Massive left ventricular hypertrophy in the following locations:
– Asymmetric hypertrophy of the interventricular septum (most frequent form with ~90 %)
– Asymmetric form with midventricular or apical hypertrophy (rare)
– Symmetrical concentric hypertrophy (rare)
– Hypertrophy of the right ventricle (rare)
 Unimpaired left ventricular systolic function with forceful, quick contractions
Unimpaired left ventricular systolic function with forceful, quick contractions
 In hypertrophic obstructive cardiomyopathy, dynamic systolic pressure gradient in the left ventricular outflow tract
In hypertrophic obstructive cardiomyopathy, dynamic systolic pressure gradient in the left ventricular outflow tract
 Markedly impaired diastolic ventricular function in terms of impaired compliance
Markedly impaired diastolic ventricular function in terms of impaired compliance
 Systolic anterior motion of the anterior mitral valve leaflet (SAM)
Systolic anterior motion of the anterior mitral valve leaflet (SAM)
 Dysplastic intramural coronary arteries and coronary arterioles
Dysplastic intramural coronary arteries and coronary arterioles
 Focal or diffuse disarray of the myofibrils and of the interstitial connective tissue
Focal or diffuse disarray of the myofibrils and of the interstitial connective tissue
Depending on the hemodynamics two forms of hypertrophic cardiomyopathy can be differentiated:
1. Hypertrophic obstructive cardiomyopathy (HOCM)
2. Hypertrophic nonobstructive cardiomyopathy (HNCM)
Characteristic for both forms of hypertrophic cardiomyopathy is impaired relaxation due to the hypertrophy and increased left ventricular stiffness with increased diastolic pressure in the left ventricle and in the left atrium. The impaired compliance leads typically to left atrial dilatation.
In contrast to HNCM, in HOCM an intraventricular obstruction or an obstruction of the left ventricular outflow tract with an associated pressure gradient develops during systole. The outflow tract obstruction is caused both by systolic protrusion of the massively hypertrophied septum and an abnormal, septal displacement of the anterior mitral valve leaflet. The high velocity with which the blood flows through the narrowed outflow tract, causes an additional suction effect, which makes the mitral leaflet move toward the septum and which therefore further narrows the outflow tract (Venturi effect, SAM phenomenon). Furthermore, in ~30 % of cases mid- to late systolic mitral regurgitation develops.
Specific Hemodynamics
HOCM with asymmetrical septal hypertrophy is characterized by a systolic pressure gradient in the left ventricular outflow tract. The pressure gradient always depends on the dynamics of the left ventricular contraction as well as preload and left ventricular afterload. Accordingly, the measured gradient can vary markedly in the same person. Therefore, in many patients with HOCM a gradient cannot be detected at rest but only manifests itself after provocation tests. In ~15 % of patients an obstruction can be detected not only in the left ventricle but also in the right ventricle. In some patients, the pressure gradient can be localized not in the subaortic region but in the midcavity or apex.
 Thereis no clear correlation between pressure gradient and clinical symptoms!
Thereis no clear correlation between pressure gradient and clinical symptoms!
Cardiac output at rest and under stress is usually normal. The ejection fraction, too, is either normal or increased at rest.
In the overwhelming majority of patients left ventricular end-diastolic pressure and thus also pulmonary capillary wedge pressure at rest are already mildly to moderately increased, with pathological increase of the PCW pressure under stress in almost all patients. The increased left ventricular end-diastolic pressure is a result of impaired compliance, and it is independent of the pressure gradient. As a result of the impaired compliance there is left atrial enlargement.
Another cause of the PCW pressure increase in patients with HOCM can be concomitant mitral regurgitation.
By definition the specific hemodynamics of HNCM are differentiated from HOCM by the total absence (i.e., including after provocation) of an intracavitary pressure gradient. Otherwise there are the same hemodynamic findings of impaired compliance as in HOCM. The hypertrophy can be distributed symmetrically or asymmetrically and also affect the right ventricle. Myocardial contractility is not impaired, similarly to HOCM.
 Indication
Indication
The disease is primarily diagnosed by echocardiography, which also allows a differentiation between obstructive and nonobstructive forms with determination of the pressure gradient; detection of a concomitant mitral regurgitation is also possible. When the findings are ambiguous, additional provocations to increase the gradient can be added (e.g., with nitrates, Valsalva maneuver, or physical stress).
Cardiac MRI is a safe diagnostic procedure to assess left ventricular mass, regional distribution of hypertrophy, left ventricular volumes, and hemodynamic changes in left ventricular outflow tract and at the mitral valve. In addition, cardiac MRI shows myocardial structure and can indicate intramyocardial fibrosis (delayed enhancement).
To functionally classify severity, there are examinations with pharmacological and nonpharmacological provocation during echocardiography and cardiac catheterization.
Cardiac catheterization is indicated in the following circumstances:
 When a reliable diagnosis by color Doppler echocardiography or other noninvasive imaging modalities is not possible
When a reliable diagnosis by color Doppler echocardiography or other noninvasive imaging modalities is not possible
 When it is planned to carry out a provocation test
When it is planned to carry out a provocation test
 When there is the possibility of CAD because of the clinical features of HCM (angina, dyspnea) and a coronary angiography should be performed
When there is the possibility of CAD because of the clinical features of HCM (angina, dyspnea) and a coronary angiography should be performed
 When a relevant mitral regurgitation is suspected
When a relevant mitral regurgitation is suspected
 When surgical therapy of HOCM is planned
When surgical therapy of HOCM is planned
 When transcoronary ablation of the septal hypertrophy in HOCM is planned
When transcoronary ablation of the septal hypertrophy in HOCM is planned
 Goals
Goals
 Quantification or exclusion of a manifest or latent intracavitary pressure gradient
Quantification or exclusion of a manifest or latent intracavitary pressure gradient
 Localization of hypertrophy
Localization of hypertrophy
 Evaluation of systolic and diastolic ventricular function
Evaluation of systolic and diastolic ventricular function
 Evaluation for concomitant mitral regurgitation
Evaluation for concomitant mitral regurgitation
 Evaluation for coronary artery disease
Evaluation for coronary artery disease
 Evaluation of the coronary anatomy to identify septal branches
Evaluation of the coronary anatomy to identify septal branches
 Procedure
Procedure
 Placement of a 5F to 6F sheath in the femoral artery and a 6F sheath in the femoral vein
Placement of a 5F to 6F sheath in the femoral artery and a 6F sheath in the femoral vein
 Catheterization of the left ventricle with placement of a double-lumen 6F pigtail catheter (distance between lumina of 5 cm), if available, otherwise normal singlelumen 5F pigtail catheter
Catheterization of the left ventricle with placement of a double-lumen 6F pigtail catheter (distance between lumina of 5 cm), if available, otherwise normal singlelumen 5F pigtail catheter
 Right heart catheterization with placement of the catheter in PCW position (balloon catheter)
Right heart catheterization with placement of the catheter in PCW position (balloon catheter)
 Simultaneous pressure recording PCW/LV
Simultaneous pressure recording PCW/LV
 Determination of cardiac output (Fick or thermodilution)
Determination of cardiac output (Fick or thermodilution)
 Simultaneous pressure recording in the left ventricle (LV/LV, alternative LV/aorta), triggering of extrasystoles during the pressure registration by catheter movement against the ventricular wall
Simultaneous pressure recording in the left ventricle (LV/LV, alternative LV/aorta), triggering of extrasystoles during the pressure registration by catheter movement against the ventricular wall
 Ventriculography (LAO projection, ideally lateral 90°)
Ventriculography (LAO projection, ideally lateral 90°)
 Calibration and left ventricular volume measurement if mitral regurgitation is detected (sphere)
Calibration and left ventricular volume measurement if mitral regurgitation is detected (sphere)
 Provocation test with repeated simultaneous pressure recording (LV/LV, alternatively LV/aorta)
Provocation test with repeated simultaneous pressure recording (LV/LV, alternatively LV/aorta)
 Left heart catheter pullback (LV–aorta) with pressure recording
Left heart catheter pullback (LV–aorta) with pressure recording
 Aortography
Aortography
 Right heart catheter pullback with pressure recording (PCW–PA–RV–apex–RA)
Right heart catheter pullback with pressure recording (PCW–PA–RV–apex–RA)
 Right ventriculography
Right ventriculography
 Coronary angiography
Coronary angiography
 Findings on Cardiac Catheterization
Findings on Cardiac Catheterization
Left Ventriculography
Characteristic for HOCM is a systolic protrusion of the hypertrophied interventricular septum with narrowing of the left ventricular outflow tract (40–60°LAO projection). Frequently the papillary muscles are hypertrophied and the inner contours of the left ventricle appear irregular due to prominent trabeculae. Furthermore, in the LAO projection the systolic anterior motion of the anterior mitral valve leaflet (SAM, Venturi effect) can be detected (Fig. 15.2). In addition, the left ventriculogram can show a concomitant mitral regurgitation, which is usually mild (if required additional lateral projection).
In HNCM the hypertrophy is localized predominantly in the mid- and apical region of the left ventricle. As the hypertrophy is more pronounced in the apex, there is a typical funnel-like constriction of the apex, which can be seen both in systole and in diastole (spade shape, Fig. 15.3). The septum, too, can protrude during systole, but this does not lead to obstruction of the left ventricular outflow tract. Usually there is no mitral regurgitation.
Coronary Angiography
Characteristic for both HOCM and HNCM is the systolic compression of septal branches of the LAD or a large diagonal (“milking” phenomenon). Furthermore, a sawfish like systolic narrowing of the proximal or middle third of the LAD as a possible sign of HCM has been described.
Hemodynamics/Provocation Tests
In the left ventricle in HOCM there is a systolic pressure difference across the obstruction. Most suitable to detect this pressure difference is simultaneous intraventricular pressure measurement with a double-lumen pigtail catheter. Alternatively, the pressure gradient can be detected by slow catheter pullback from the apex to the aorta (Fig. 15.4). If there is no pressure gradient at rest, it is tested whether a pressure gradient occurs after provocation. The following provocation tests are suitable:
Fig. 15.2 a, b Left ventriculogram in hypertrophicobstructive cardiomyopathy (HOCM).
a RAO projection: pronounced concentric hypertrophy with systolic encompassing of the pigtail catheter, concomitant mitral regurgitation.
b LAO projection: systolic obstruction of the left ventricular outflow tract with protrusion of the anterior mitral valve leaflet to the septum (Venturi effect).
Fig. 15.3 Left ventriculogram in hypertrophic non-obstructive cardiomyopathy (HNCM). Typical funnel-shaped constriction of the apex (spade form).
 Postextrasystolic potentiation (Brockenbrough phenomenon)
Postextrasystolic potentiation (Brockenbrough phenomenon)
 Valsalva maneuver
Valsalva maneuver
 Nitroglycerin (sublingual or IV)
Nitroglycerin (sublingual or IV)
 Orciprenalin IV
Orciprenalin IV
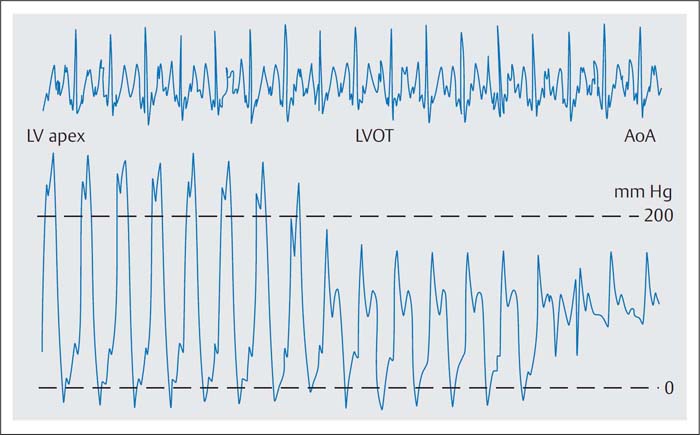
Fig. 15.4 Catheter pullback LV-apex–LV-outflow tract (LVOT)–aorta with detection of an intracavitary pressure gradient of 100 mm Hg in HOCM.
The Brockenbrough phenomenon is characteristic for HOCM. Due to postextrasystolic potentiation of myocardial contractility the heartbeat that follows the extrasystole is normally associated with a systolic pressure rise in the left ventricle and in the aorta because of the larger stroke volume. In contrast, in HOCM the extrasystole results in increased obstruction with an increased intraventricular pressure gradient and reduction of the aortic pressure. This so-called Brockenbrough phenomenon is deliberately provoked by the operator by touching the ventricular wall with the distal catheter and it serves to differentiate between the obstructive and the nonobstructive forms of HCM (Fig. 15.5).
With continuous recording, ventricular pressures are measured simultaneously with a double-lumen pigtail catheter to record the intracavitary pressure gradient. Alternatively, ventricular and aortic pressures (singlelumen pigtail catheter in the ventricle, aortic pressure via the side arm of the arterial sheath) are measured simultaneously to record the postextrasystolic reduction in systolic aortic pressure.
Additional provocation tests are the Valsalva maneuver and administration of nitrates, both of which increase the obstruction via a reduced left ventricular filling.
To reliably detect or exclude an obstructive form, a pharmacological provocation test
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree