CARDIAC TRANSPLANTATION AND PROLONGED ASSISTED CIRCULATION
Advanced or end-stage heart failure is an increasingly frequent sequela, as progressively more effective palliation for the earlier stages of heart disease and prevention of sudden death associated with heart disease become more widely recognized and employed (Chap. 17). When patients with end-stage or refractory heart failure are identified, the physician is faced with the decision of advising compassionate end-of-life care or choosing to recommend extraordinary life-extending measures. For the occasional patient who is relatively young and without serious comorbidities, the latter may represent a reasonable option. Current therapeutic options are limited to cardiac transplantation (with the option of mechanical cardiac assistance as a “bridge” to transplantation) or (at least in theory) the option of permanent mechanical assistance of the circulation. In the future, it is possible that genetic modulation of ventricular function or cell-based cardiac repair will be options for such patients. Currently, both approaches are considered to be experimental.
CARDIAC TRANSPLANTATION
Surgical techniques for orthotopic transplantation of the heart were devised in the 1960s and taken into the clinical arena in 1967. The procedure did not gain widespread clinical acceptance until the introduction of “modern” and more effective immunosuppression in the early 1980s. By the 1990s, the demand for transplantable hearts met, and then exceeded, the available donor supply and leveled off at about 4000 heart transplants annually worldwide, according to data from the Registry of the International Society for Heart and Lung Transplantation (ISHLT). Subsequently, heart transplant activity in the United States has remained stable at ~2200/year, but worldwide activity reported to this registry has decreased somewhat. This apparent decline in numbers may be a result of the fact that reporting is legally mandated in the United States, but not elsewhere, and several countries have started their own databases.
SURGICAL TECHNIQUE
Donor and recipient hearts are excised in virtually identical operations with incisions made across the atria and atrial septum at the midatrial level (leaving the posterior walls of the atria in place) and across the great vessels just above the semilunar valves. The donor heart is generally “harvested” in an anatomically identical manner by a separate surgical team and transported from the donor hospital in a bag of iced saline solution and then is reanastomosed into the waiting recipient in the orthotopic or normal anatomic position. The only change in surgical technique since this method was first described has been a movement in recent years to move the right atrial anastamosis back to the level of the superior and inferior vena cavae to better preserve right atrial geometry and prevent atrial arrhythmias. Both methods of implantation leave the recipient with a surgically denervated heart that does not respond to any direct sympathetic or parasympathetic stimuli but does respond to circulating catecholamines. The physiologic responses of the denervated heart to the demands of exercise are atypical but quite adequate to carry on normal physical activity.
DONOR ALLOCATION SYSTEM
In the United States, the allocation of donor organs is accomplished under the supervision of the United Network for Organ Sharing (UNOS), a private organization under contract to the federal government. The United States is divided geographically into eleven regions for donor heart allocation. Allocation of donor hearts within a region is decided according to a system of priority that takes into account (1) the severity of illness, (2) geographic distance from the donor, and (3) patient time on the waiting list. A physiologic limit of ~3 h of “ischemic” (out-of-body) time for hearts precludes a national sharing of hearts. This allocation system design is reissued annually and is responsive to input from a variety of constituencies, including both donor families and transplant professionals.
At the current time, highest priority according to severity of illness is assigned to patients requiring hospitalization at the transplant center for IV inotropic support with a pulmonary artery catheter in place for hemodynamic monitoring or to patients requiring mechanical circulatory support (i.e., intraaortic balloon pump [IABP], right or left ventricular assist device [RVAD, LVAD], extracorporeal membrane oxygenation [ECMO], or mechanical ventilation). Second highest priority is given to patients requiring ongoing inotropic support, but without a pulmonary artery catheter in place. All other patients have priority according to their time accrued on the waiting list, and matching is achieved only according to ABO blood group compatibility and gross body size compatibility, although some patients who are “presensitized” and have preexisting anti-HLA antibodies (commonly multiparous women or patients previously multiply transfused) undergo prospective cross-matching with the donor. While HLA matching of donor and recipient would be ideal, the relatively small numbers of patients, as well as the time constraints involved, make such matching impractical.
INDICATIONS/CONTRAINDICATIONS
Heart failure is an increasingly common cause of death, particularly in the elderly. Most patients who reach what has recently been categorized as stage D, or refractory end-stage heart failure, are appropriately treated with compassionate end-of-life care. A subset of such patients who are younger and without significant comorbidities can be considered as candidates for heart transplantation. Exact criteria vary in different centers but generally take into consideration the patient’s physiologic age and the existence of comorbidities such as peripheral or cerebrovascular disease, obesity, diabetes, cancer, or chronic infection.
RESULTS
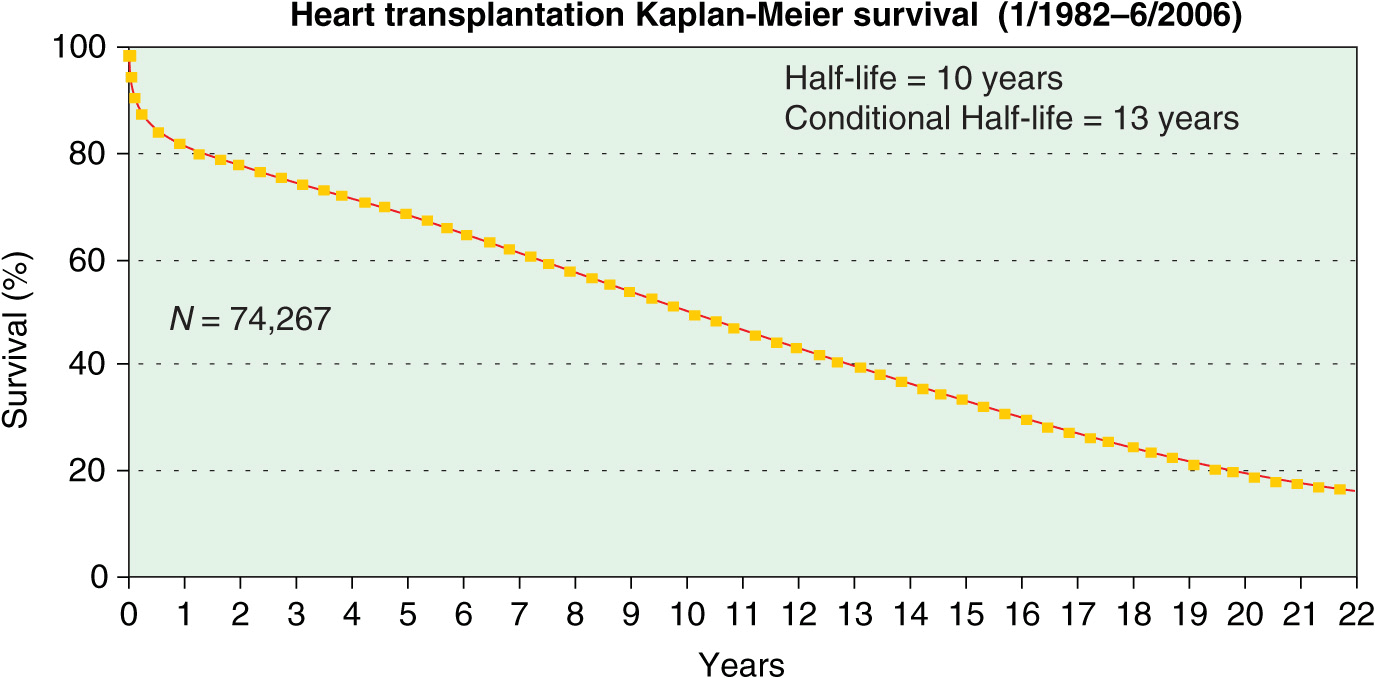
A registry organized by the ISHLT has tracked worldwide and U.S. survival rates after heart transplantation since 1982. The most recent update reveals 83% and 76% survival 1 and 3 years posttransplant, or a posttransplant “half-life” of 10 years (Fig. 18-1). The quality of life in these patients is generally excellent, with well over 90% of patients in the registry returning to normal and unrestricted function following transplantation.
FIGURE 18-1
Survival was calculated using the Kaplan-Meier method, which incorporates information from all transplants for whom any follow-up has been provided. Because many patients are still alive and some patients have been lost to follow-up, the survival rates are estimates rather than exact rates because the time of death is not known for all patients. Therefore, 95% confidence limits are provided. (From J Heart Lung Transplant 2008; 27:937–983.)
IMMUNOSUPPRESSION
Medical regimens employed to provide suppression of the normal immune response to a solid organ allograft vary from center to center and are in a constant state of evolution, as more effective agents with improved side-effect profiles and less toxicity are introduced. All currently used regimens are nonspecific, providing general hyporeactivity to foreign antigens rather than donor-specific hyporeactivity, and also providing the attendant, and unwanted, susceptibility to infections and malignancy. Most cardiac transplant programs currently use a three-drug regimen including a calcineurin inhibitor (cyclosporine or tacrolimus), an inhibitor of T cell proliferation or differentiation (azathioprine, mycophenolate mofetil, or sirolimus), and at least a short initial course of glucocorticoids. Many programs also include an initial “induction” course of polyclonal or monoclonal anti-T cell antibodies in the perioperative period to decrease the frequency or severity of early posttransplant rejection. Most recently introduced have been monoclonal antibodies (daclizumab and basiliximab) that block the interleukin 2 receptor and may provide prevention of allograft rejection without additional global immunosuppression.
Diagnosis of cardiac allograft rejection is usually made with the use of endomyocardial biopsy, either done on a surveillance basis or in response to clinical deterioration. Biopsy surveillance is performed on a regular basis in most programs for the first year postoperatively and for the first 5 years in many programs. Therapy consists of augmentation of immunosuppression, the intensity and duration of which is dictated by the severity of the rejection.
LATE POSTTRANSPLANT MANAGEMENT ISSUES
Increasing numbers of heart transplant patients are surviving for years following transplantation and constitute a population of patients with a number of long-term management issues.
Allograft coronary artery disease
Despite usually having young donor hearts, cardiac allograft recipients are prone to develop coronary artery disease (CAD). This CAD is generally a diffuse, concentric, and longitudinal process that is quite different from “ordinary” atherosclerotic CAD, which is more focal and often eccentric. The underlying etiology is most likely primarily immunologic injury of the vascular endothelium, but a variety of risk factors influence its existence and progression and include nonimmunologic factors such as dyslipidemia, diabetes mellitus, and cytomegalovirus (CMV) infection. It is hoped that newer and improved immunosuppressive modalities will reduce the incidence and impact of these devastating complications, which currently account for the majority of late posttransplant deaths. Thus far, the immunosuppressive agents mycophenolate mofetil and the mammalian target of rapamycin (mTOR) inhibitors sirolimus and everolimus have been shown to be associated with short-term lesser incidence and extent of coronary intimal thickening; in anecdotal reports, institution of sirolimus was associated with some reversal of the disease. The use of statins has also been shown to be associated with a reduced incidence of this vasculopathy, and these drugs are now almost universally used in transplant recipients unless contraindicated. Palliation of the disease with percutaneous interventions is probably safe and effective in the short term, although the disease often advances relentlessly. Because of the denervated status of the organ, patients rarely experience angina pectoris, even with advanced stages of the disease.
Retransplantation is the only definitive form of therapy for advanced allograft CAD, but the scarcity of donor hearts makes the decision to pursue retransplantation a difficult one in an individual patient, as well as a difficult ethical issue.
Malignancy
The occurrence of an increased incidence of malignancy is a well-recognized sequela of any program of chronic immunosuppression, and organ transplantation is no exception. Lymphoproliferative disorders are among the most frequent posttransplant complications and, in most cases, seem to be driven by the Epstein-Barr virus. Effective therapy includes reduction of immunosuppression (a clear “double-edged sword” in the setting of a life-sustaining organ), antiviral agents, and traditional chemo- and radiotherapy. Most recently, specific antilymphocyte (CD20) therapy has shown great promise. Cutaneous malignancies (both basal cell and squamous cell carcinomas) also occur with increased frequency in transplant recipients and can pursue very aggressive courses. The role of decreasing immunosuppression for treatment of these cancers is far less clear.
Infections
The use of currently available nonspecific immunosuppressive modalities to prevent allograft rejection naturally results in an increased susceptibility to infectious complications in transplant recipients. Although their incidence has decreased since the introduction of cyclosporine, infections with unusual and opportunistic organisms remain the major cause of death during the first postoperative year and remain a threat to the chronically immunosuppressed patient throughout life. Effective therapy depends on careful surveillance for early signs and symptoms of opportunistic infection and an extremely aggressive approach to obtaining a specific diagnosis as well as expertise in recognizing the more common clinical presentations of CMV, Aspergillus, and other opportunistic infectious agents.
PROLONGED ASSISTED CIRCULATION
The modern era of mechanical circulatory support can be traced back to 1953, when cardiopulmonary bypass was first used in a clinical setting and ushered in the possibility of brief periods of circulatory support to permit open-heart surgery. Subsequently, a variety of extracorporeal pumps to provide circulatory support for brief periods of time have been developed. The use of a mechanical device to support the circulation for more than a few hours initially developed slowly, with the implant of a total artificial heart in 1969 in Texas by Cooley. This patient survived for 60 h until a donor organ became available, at which point he was transplanted. Unfortunately, the patient died of pulmonary complications after transplantation. The entire field of mechanical replacement of the heart took a decade-long hiatus until the 1980s, when total artificial hearts were reintroduced with much publicity; however, they failed to produce the hoped-for treatment of end-stage heart disease. Starting in the 1970s, parallel to the development of the total artificial heart, there was intense research in the development of ventricular assist devices, which provide mechanical assistance to (rather than replacement of) the failing ventricle (currently, newer versions of the total artificial heart are in preliminary clinical trials).
Although conceived of initially as alternatives to biologic replacement of the heart, LVADs were introduced as, and are still employed primarily as, temporary “bridges” to heart transplantation in candidates who begin to fail medical therapy before a donor heart becomes available. Several devices are approved by the U.S. Food and Drug Administration (FDA) and are currently in widespread use. Those that are implantable within the body are compatible with hospital discharge and offer the patient a chance for life at home while waiting for a donor heart. However successful such “bridging” is for the individual patient, it does nothing to alleviate the scarcity of donor hearts; the ultimate goal in the field remains that of providing a reasonable alternative to biologic replacement of the heart—one that is widely and easily available and cost-effective.
CURRENT INDICATIONS AND APPLICATIONS OF VENTRICULAR ASSIST DEVICES
Currently, there are two major indications for long-term ventricular assistance. First, patients with chronic end-stage heart failure are eligible for mechanical support if they are at risk of imminent death from cardiogenic shock. Second, if patients have a left ventricular ejection fraction <25%, peak VO2 <14 mL/kg/min, or are dependent on inotropic therapy or support with intraaortic balloon counterpulsation, they may be eligible for mechanical support. If they are eligible for heart transplantation, the mechanical circulatory assistance is termed “bridge to transplantation.” By contrast, if the patient has a contraindication to heart transplantation, the device therapy is deemed to be “destination” left ventricular assistance therapy.
AVAILABLE DEVICES
In the United States, there are currently four FDA-approved devices that are used as bridges to transplantation in adults. Of these four devices, one is also approved for use as destination therapy or long-term mechanical support of the heart. There are a number of other devices that are approved only for short-term support for post-cardiac surgery shock or for patients with cardiogenic shock secondary to acute myocardial infarction or fulminant myocarditis; these will not be considered here. None of the long-term devices as yet are totally implantable and, because of this need for transcutaneous connections, all share a common problem with infectious complications. Likewise, all share some tendency to thromboembolic complications as well as the expected possibility of mechanical device failure common to any machine.
The CardioWest total artificial heart (TAH) (Syncardia, Tucson, AZ) is a pneumatic, biventricular, orthotopically implanted total artificial heart with an externalized driveline connecting it to its console. It consists of two spherical polyurethane chambers with polyurethane diaphragms. Inflow and outflow conduits are constructed of Dacron and contain Medtronic-Hall (Medtronic, Inc., Minneapolis, MN) valves. It is currently the only FDA-approved device for use as a bridge to transplantation in patients who have severe biventricular failure.
The Thoratec LVAD (Thoratec Corp., Pleasanton, CA) is an extracorporeal pump that takes blood from a large cannula placed in the left ventricular apex and propels it forward through an outflow cannula inserted into the ascending aorta. The pump itself sits in the paracorporeal position on the abdomen and is attached to a device console cart with wheels, allowing for limited ambulation. The extracorporeal nature of this pump allows it to be used in small adults for whom intracorporeal pumps would be too large.
The Novacor LVAD (WorldHeart, Inc., Oakland, CA) also takes blood from the left ventricular apex through a cannula and propels it into the ascending aorta through a second cannula. With this device, the pump itself is placed in a surgically created pocket in the peritoneal fascia in the abdomen. A driveline that connects to the power source is tunneled subcutaneously and usually exits in the right upper quadrant of the abdomen.
The HeartMate XVE LVAD (Thoratec Corp., Pleasanton, CA) is an intracorporeal left ventricular assist device that has an externalized driveline. The pump sits in the anterior abdominal wall with cannulae that traverse across the diaphragm. There is a drainage cannula in the left ventricular apex, and the blood is expelled from the pump into the ascending aorta via a synthetic vascular graft. This device may be used as a bridge to transplantation and patients may be discharged from the hospital with this device to await transplantation. The HeartMate XVE LVAD is now one of two FDA-approved devices for destination therapy.
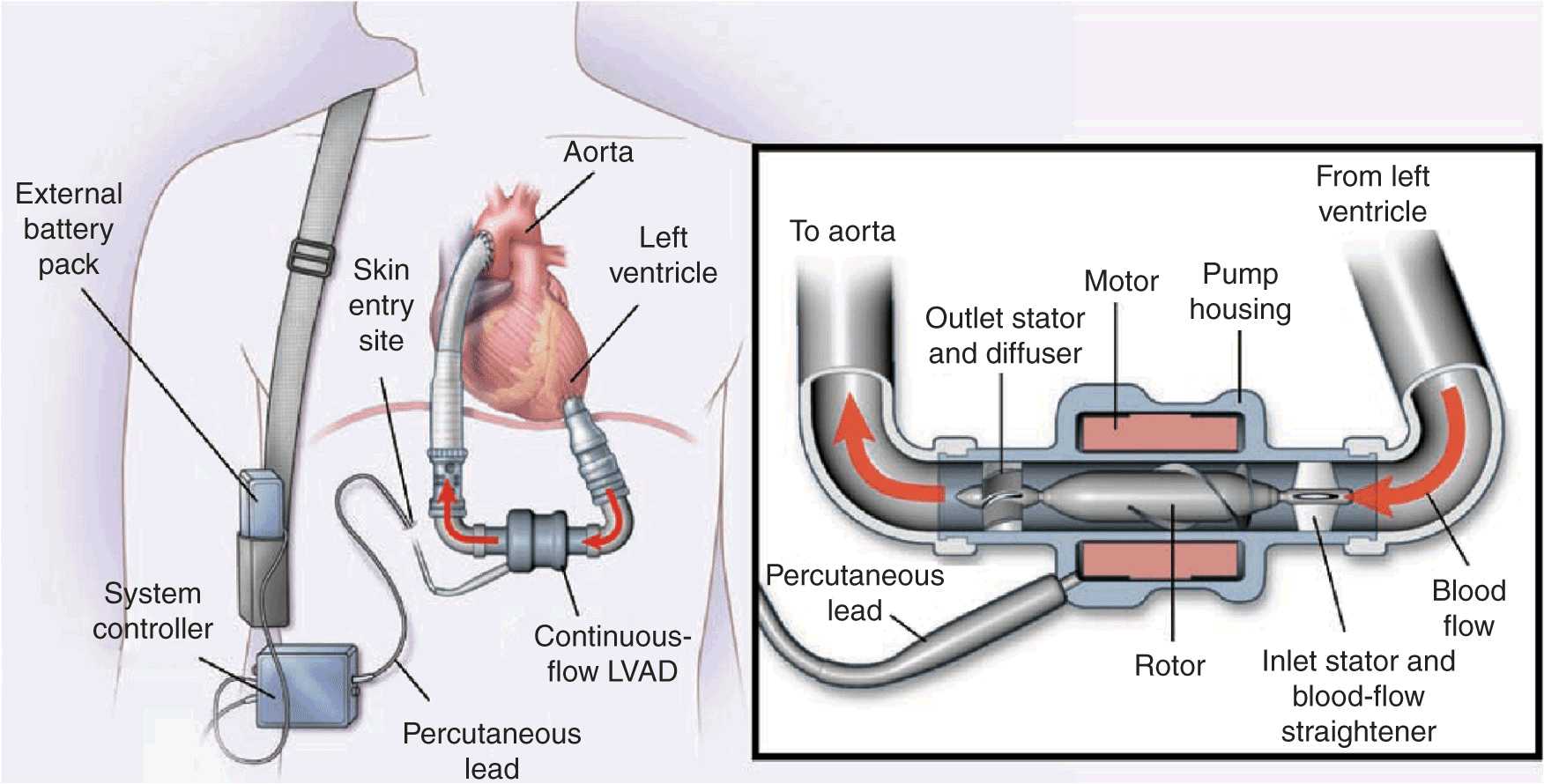
The HeartMate II LVAS (Thoratec Corp., Pleasanton, CA) similarly uses a drainage cannula in the left ventricular apex to drain blood into a small chamber, where the blood is driven by an electrically powered motor that spins a rotor, accelerating blood outflow into the ascending aorta (Fig. 18-2). This device is currently the only FDA-approved axial-flow pump that can be used both as a bridge to transplantation and as destination therapy. There are several other axial-flow pumps currently being investigated. These devices have fewer moving parts than previous devices and provide nonpulsatile blood flow. All current axial-flow pumps continue to require transcutaneous connections to power the electric motor. Newer, third-generation devices, which also provide nonpulsatile flow, work through a different mechanism than the axial-flow pumps and are currently being investigated. These devices are even smaller than the currently available axial-flow pumps, and their mechanism of action involves less trauma to blood cells, which may result in better durability and decreased long-term complications.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree