Fig. 37.1
Amplatzer Septal Occluder device. (A) Right atrial angiogram performed after deployment of the device in a secundum atrial septal defect, but before release. The right atrial disc is obscured by contrast with the waist within the atrial septal defect. (B) The levophase of the right atrial angiogram opacifying the left atrium. Contrast outlines the left atrial disc completely within the left atrium
37.5.1 Animal Testing of the Amplatzer Device Designs
The Amplatzer Septal Occluder device was originally designed for occlusion of a secundum ASD. Initial animal studies focused on reproducibly creating such atrial septal communications, as a natural model of a secundum ASD did not exist. To do so, nonsurgically the flap of the foramen ovale in the experimental animal was perforated and dilated with a balloon to induce an atrial communication [6]; subsequently, devices were implanted, and, in most cases, there were no complications or residual leaks. It was important to show that no thrombus formed on the devices. Afterwards, human clinical trials confirmed that no retroaortic rim was required for stable device position and complete closure. Importantly, patients could be discharged the morning after device placement, and they remained on low dose aspirin and endocarditis prophylaxis for 6 months after closure [16].
37.5.2 Required Testing for FDA Approval
An FDA-approved study to provide clinical evidence of the effectiveness of the Amplatzer Septal Occluder was originally initiated, based on the prior success of animal studies and European trials in humans. Yet, the clinical study design for this device was considered difficult. In general, patients and their families wanted to avoid surgery, despite the long history of safe surgical closure and the lack of long-term follow-up with this new device. Therefore, a blinded randomization was unsuccessful, as many patients and families that were chosen for the surgical group simply opted out of the trial, preferring to wait for final FDA approval . The overall study design was subsequently modified to allow device closure at some institutions with patients recruited to designated surgical centers. This is not true randomization, but is representative of the difficulty of study design in the real world.
Later, the results of Phase II of the FDA trial also showed that the Amplatzer Septal Occluder was an effective and safe therapy as compared to the surgical group. Importantly, at the end of 12 months, there was complete closure or a small (<2 mm) residual shunt in 98.5 % of device patients, compared to 100 % of surgically closed patients. Furthermore, there were no differences between groups in the incidence of major complications. Minor complications were more common in surgical patients (27/442, 6.1 % versus 29/154, 18.8 %); however, one needs to consider that all patients were not truly randomized. There were differences between groups, with the surgical patients being younger (18.1 ± 19.3 versus 5.9 ± 6.2 years, p < 0.001) and smaller (42.3 ± 27.3 kg versus 20.6 ± 15.2 kg, p < 0.001) [16]. In December 2001, the FDA granted premarket approval of the Amplatzer Septal Occluder, the first ASD closure device with such an approval.
37.5.3 Continued Animal Research and Translation to Humans
After extensive successful clinical use, several reports of perforation of the heart by the device began to appear in a small number of patients with secundum ASDs [17]. Subsequently, a careful review of information from patients suffering from erosion after Amplatzer Septal Occluder placement suggested that individuals with “deficiency” of the superior and anterior rims of the atrial septum were at highest risk of this complication (Fig. 37.2). In fact, the majority of patients with secundum ASDs have very little retroaortic rims, so many patients with ASDs may be at increased risk of erosions of the Amplatzer Septal Occluder through the superior, anterior left, or right atrial walls. This anatomical relationship of the ascending aorta to the anterior rim of the secundum ASD was not fully appreciated until these complications of erosion were reported. It should also be noted that the original animal model studied did not truly mimic the anatomies of many patients with secundum ASDs. More recently, using stop flow to eliminate oversizing of the Amplatzer Septal Occluder device [17] has reduced the frequency of these complications (personal communications with Ken Lock, St. Jude Medical Corporation).
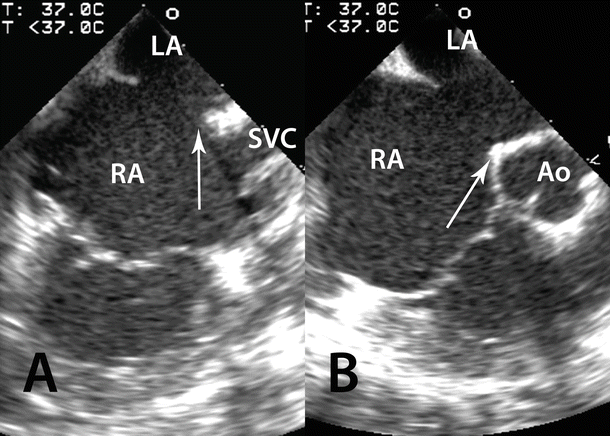

Fig. 37.2
Transesophageal images of an atrial septal defect with minimal superior and anterior (retroaortic) rims. (A) A bicaval view is recorded with almost no rim (arrow) by the superior vena cava (SVC). (B) A short axis view of the aorta (Ao) is recorded with minimal retroaortic rim (arrow). Atrial septal defects with minimal rims in these two areas are likely to bring the edges of right or left atrial retention discs in contact with the right or left atrial wall against the ascending aorta and may have a higher risk of cardiac perforation. LA left atrium, RA right atrium
37.6 Patent Ductus Arteriosus
A patent ductus arteriosus is a failure of closure of a vascular channel present in the fetus that normally closes within the first few days after birth. When this vessel remains open, overcirculation of the lungs results. This, in turn, can damage the pulmonary vasculature, overwork the heart, and predispose these individuals to infective endocarditis. Closure of this channel is recommended to reduce the workload of the heart, when spontaneous closure is no longer likely (beyond 1–2 years of age) [18]. Much like operative closure of a secundum ASD, surgical closure of a PDA is a low risk procedure that has been used for decades [19]. Therefore, any attempt to employ transcatheter closure methods must carry a procedural risk at least as low as surgery. Furthermore, a PDA is similar to a secundum atrial defect in that the vascular communication is surrounded by normal vessels. It has been shown that concentric devices modified from the design of the Amplatzer Septal Occluder provided the opportunity for transcatheter closure procedures in patients with these defects.
Successful transcatheter closure of a small PDA was available before a specific closure device was developed. More specifically, coil occlusion of a PDA was first performed at the University of Minnesota in 1972. These early procedures included filling the aortic ampulla with stainless steel coils and their attached Dacron fibers, or “hanging” a coil across the narrowest part of a PDA; both procedures produced reliable closure. The first embolization coils were not attached to delivery wires, and the coils sometimes embolized into the pulmonary circulation. These techniques were most effective when the narrowest diameter of the patent ductus was less than 3 mm [20]. During this period of time, a retrievable device that would occlude larger ductus defects was considered desirable.
The Amplatzer Ductal Occluder is shaped like a plug, sized to the aortic ampulla with an aortic retention disc designed to prevent embolization through the ductus (Fig. 37.3). This device is typically delivered via a venous route, and the delivery catheter is small (5–8 Fr) because of the small collapsed device diameter. This simple modification of a self-expanding stent was found to be extremely successful in producing complete occlusion of even larger PDAs. In the Phase II FDA trial, there was an observed complete closure of over 97 % of PDAs at 6 and 12 months. There was only a 2.3 % incidence of serious or major adverse events (including one embolization that required surgical removal and one death of a child, not device related, with a chromosomal trisomy) [21]. Premarket FDA approval of the Amplatzer Ductal Occluder device was received in January 2003.
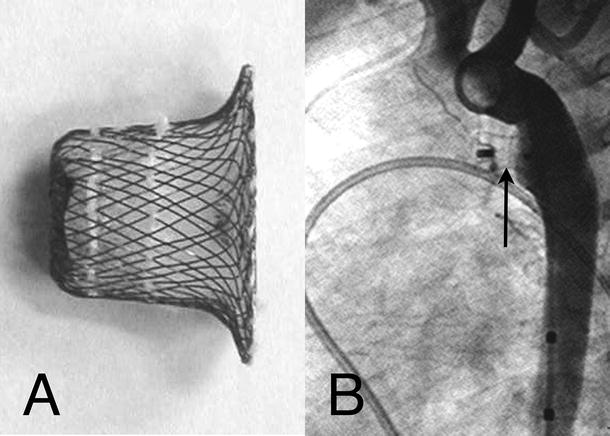

Fig. 37.3
Amplatzer Ductal Occluder device . (A) Photograph of the device with clearly visible suturing of the baffle and stuffing to the ductal plug. (B) Aortogram immediately after device placement. The aortic disc is flat against the aortic wall with the plug within the ductal lumen. There is no flow through the ductus and no obstruction of the aorta or left pulmonary artery
37.6.1 Animal Testing of the Amplatzer Ductal Occluder and Translation to Human Use
A persistent PDA does not occur reliably in animals; therefore, a model needed to be created to test the efficacy of the Amplatzer Ductal Occluder. An asynthetic tube was sewn between the descending aorta and the pulmonary artery in animals [22]. The Amplatzer Ductal Occluder device worked well in these animals, completely occluding the surgically created PDA with endothelialization within 3 months. In animal trials, there were no complications, the device was retrievable, and there were no residual hemodynamic abnormalities.
Subsequent to the successful animal studies, clinical trials were undertaken with a high rate of success in occluding various sizes of PDAs in patients [23]. Yet with more extensive clinical use, a few case reports began to surface of partial obstruction of the descending thoracic aorta by the retention disc of the Amplatzer Ductal Occluder device. This was the result of the angle (approximately 65°) of insertion of the naturally occurring PDA with the descending aorta. In these cases, the superior portion of the retention disc was shown to be angled into the aorta by the plug within the PDA (Fig. 37.4), sometimes producing partial aortic obstruction in smaller patients with larger PDAs. It should be noted that the surgically created experimental PDA in the animal model was sewn at a 90° angle to the aorta, so this complication was not predicted by the anatomically inaccurate orientation.
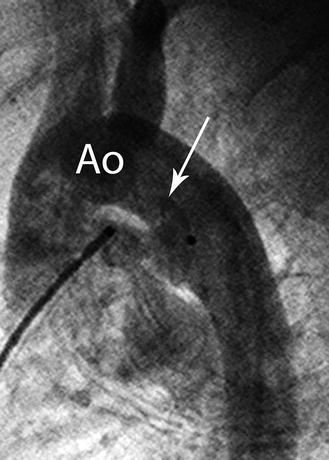

Fig. 37.4
An aortogram recorded in lateral projection after placement of an Amplatzer Ductal Occluder device, fully deployed before release. The aortic retention rim protrudes superiorly (arrow) into the aortic lumen (Ao). The angle of the human patent ductus arterosus is at an angle of approximately 65°, instead of 90° in the animal model. This device is oversized and can be retrieved using the delivery cable still attached to the device
37.6.2 Animal Trials Designed to Test Prototype Angled Amplatzer Ductal Occluder Devices
To reduce the complexity of manufacturing the Amplatzer Ductal Occluder device and, at the same time, address the issues of the angle of the descending aorta with the PDA, a new device was designed with an angled retention disc; the wire count was also increased from 72 to 144 wires, thus eliminating the need for fabric in the device [24]. For animal testing of this new design in next stage trials, a synthetic tube was sewn between the pulmonary artery and aorta at the angle of the more naturally occurring human PDA. Subsequently, in all animals in this preclinical trial, the angled Amplatzer Ductal Occluder device was successful in occluding the created PDA (Fig. 37.5A); however, the initial human use of the device revealed shortcomings with these animal trials. Specifically, an angled Amplatzer Ductal Occluder was successfully placed in a young infant with initial success, which also avoided any protrusion of the device into the aorta; however, after 3 months, the device expanded the PDA resulting in “recanalization” (Fig. 37.5B) [25]. This complication was considered to result from two unanticipated design problems. First, the hoop strength of the angled Amplatzer Ductal Occluder was sufficient to dilate the PDA in a young infant. This, in turn, resulted in an increase in the distance between the radial “spokes” of the device, with less occlusive resistance. In retrospect, the animal model trial used a non-expandable PDA with small diameters that limited the distance between the wires of the device.
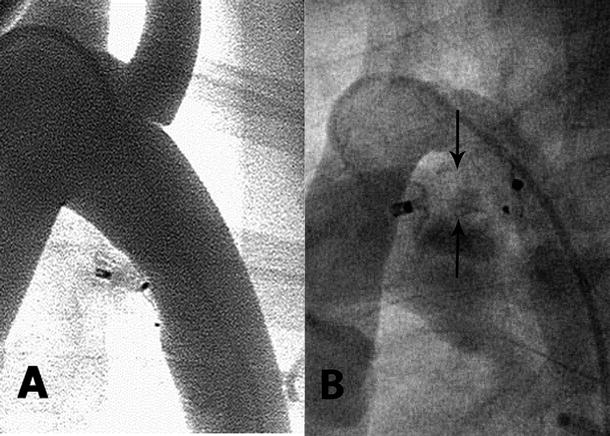

Fig. 37.5
(A) An aortogram is performed after placement and release of an angled ductal occluder device in a canine model with the artificial ductus sewn to mimic the natural angle of the human patent ductus arteriosus. The angled aortic retention disc lies flat against the aortic wall with no protrusion into the aortic lumen. The “plug” of the device is compressed with immediate complete occlusion. (B) A similar angled patent ductus arteriosus device has been placed in a human infant. The angiogram is recorded 3 months after implantation. The device plug was initially compressed with minimal flow through the middle. After 3 months, the device has expanded the ductus (arrows) with increased interwire distance and “recanalization”
37.6.3 Redesign of a Device Without Fabric and Flexible Retention Disc Orientation
The latest iteration of the Amplatzer family of ductal occluder devices (Amplatzer Ductal Occluder device 2—ADO2) is longitudinally symmetrical with retention discs on each end and with flexibility at the connection of the retention discs to the occluding plug (Fig. 37.6). This allows the retention discs to angle and lie smoothly against the aortic or pulmonary wall. To create an animal model for testing this device, infant piglets had balloon dilation of the probe PDA so that an anatomically true defect was available [26]. Subsequently, the size of the discs was further reduced to allow implantation in smaller infants (ADO2-Additional Sizes, ADO2-AS). This device was tested after ductal dilation in newborn piglets weighing 1800–2200 g and implanted under echocardiography to mimic occlusion in a premature infant in the isolette, similar to surgical ligation in this population. The animal testing was successful [27], and there are early positive results in clinical trials of premature infants outside the United States [28].
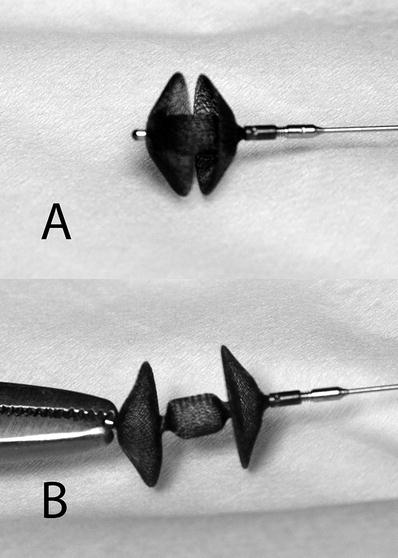

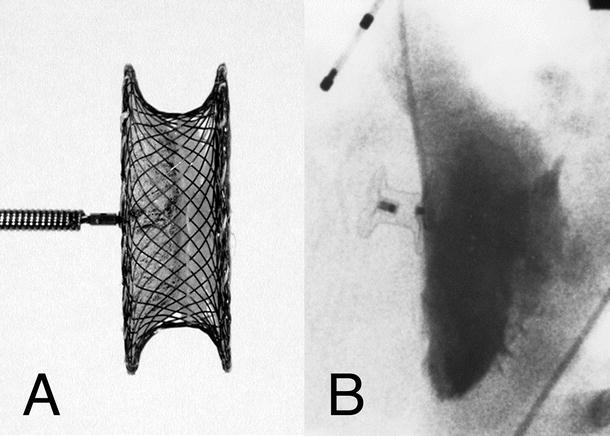
Fig. 37.6
Amplatzer Ductal Occluder II device. The device is longitudinally symmetrical allowing implantation from either the aortic or pulmonary approach. The constriction of the device between the central plug and the retention discs allows the discs to swivel and align against the vascular wall of the aorta and pulmonary artery
37.7 Muscular Ventricular Septal Defect
Muscular ventricular septal defects can occur in the lower, thicker ventricular septum. Closure of such defects is clinically recommended for the same indications as ASDs and PDAs—eliminating overwork of the heart and overcirculation of the lungs. However, unlike for the other two defects, surgery to close a muscular ventricular septal defect is generally not a simple or low risk option. More specifically, the surgical closure of muscular ventricular septal defects can be difficult at best, e.g., the right ventricular aspect of the defect can be hidden from the surgeon’s view by trabeculations within the right ventricular cavity. This, in turn, can result in a high incidence of residual leaks with a right ventricular approach. On the other hand, directly incising the left ventricle would allow clearer visualization of the defect margins, but left ventricular aneurysms or diminished left ventricular function has resulted in some cases [29]. These surgical difficulties make the transcatheter closure of such ventricular defects an attractive alternative.
In general, the Amplatzer Muscular Ventricular Septal Occluder is very similar to the Amplatzer Septal Occluder. Like a secundum ASD, muscular ventricular septal defects are separated from cardiac valves by myocardium; the obvious difference is the thickness of the ventricular myocardium. Therefore, these devices were designed with greater distances between the discs to accommodate for the differences in myocardial thickness (Fig. 37.7). Greater stability was found to be produced by the radial force applied against the thicker muscular ventricular septum, thus the retention disc diameters were decreased to 6–8 mm larger than the waist. From a design standpoint, it should be noted that initial attempts of transcatheter closure of muscular ventricular septal defects, using the Clamshell/CardioSEAL device, produced a 40 % incidence of residual leaks [30]. These devices have a central post instead of a waist the size of the defect. Therefore, a ventricular “retention” disc had to be sized at least twice the diameter of the defect; residual leaks could result from migration of the central post within the defect. In contrast, the self-centering Amplatzer Muscular Ventricular Septal Occluder is fixed within the defect by its waist. Another advantage of the Amplatzer device is the smaller maximum device diameter required to close a muscular ventricular septal defect, compared with central post devices.
 < div class='tao-gold-member'>
< div class='tao-gold-member'>





Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


