Fig. 31.1
Illustration of an implanted CRT system with right atrial, right ventricular, and left ventricular leads. Courtesy of Medtronic plc
31.4 Implantation of CRT
Implantation of a CRT system typically involves delivery of an RA lead, an RV lead, and an LV lead. The RV lead is typically positioned first to allow backup pacing if the AV node is temporarily blocked during CS cannulation and delivery of the LV lead. To date, the RV apex has been the most common implant site, but other locations such as the RV septum are also used. The first step for implanting the LV lead is cannulation of the CS. A combination of an outer sheath with or without a guidewire may be used, as well as an electrophysiology (EP) catheter . An EP catheter may also be used with recording of EGMs for guidance. Once CS access is obtained, an occlusive coronary venous angiography may be performed by introducing a balloon catheter through the delivery sheath, to determine coronary veins suitable for LV lead delivery. Venography is often recorded with cine fluoroscopy in one or two views (Fig. 31.2). Some implanters may only “puff” contrast through the sheath, or not introduce contrast at all out of concern for toxicity in patients with poor kidney function or to reduce the number of steps in the procedure [39]. The LV lead can then be delivered via stylet or guidewire to the target vein. Telescoping catheter systems consisting of an inner and outer sheath can also be used to aid in the delivery of the LV lead with greater support (Fig. 31.3). These systems may reduce the number of failed implants, improve LV lead implant location, and shorten LV positioning time [40]. A lateral or posterolateral vein is often preferred for implant, based on studies that suggest these branches are more likely to be beneficial for CRT therapy than anterior positions [41, 42]. Implanters also prefer to avoid the apex for permanent LV pacing, as this location has been associated with inferior outcomes in studies such as MADIT-CRT [43]. Once a stable position is attained, electrical testing for acceptable pacing capture thresholds and phrenic nerve stimulation (PNS) is performed. An LV pacing capture threshold ≤2.5 V, and a difference (i.e., margin) between PNS threshold and LV capture threshold of at least 3 V, is preferred to avoid complications [44]. Note that the potential for PNS is often tested by pacing at maximum outputs (e.g., 10 V). Multiple vectors may be tested to find an acceptable configuration before resorting to repositioning the lead, i.e., when such leads have multiple pacing electrodes (see below). The delivery catheter(s) are removed after the lead position is deemed acceptable. Removal requires slitting the length of the sheath using a slitting tool or separating the sheath by hand in the case of peelable catheters. The LV lead thresholds are often retested prior to insertion into the pacing device to ensure that electrical performance has not deteriorated during removal of the implant tools. If an LV lead cannot be implanted via the transvenous approach, a common alternative is to place an epicardial lead surgically, if the procedure can be tolerated by the patient.
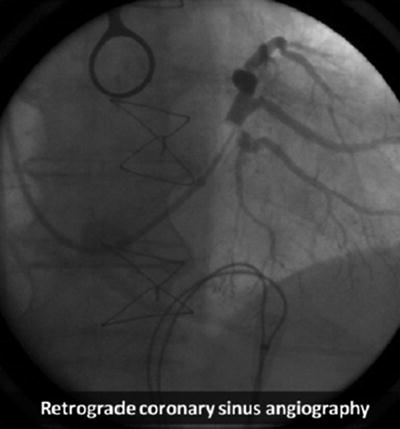
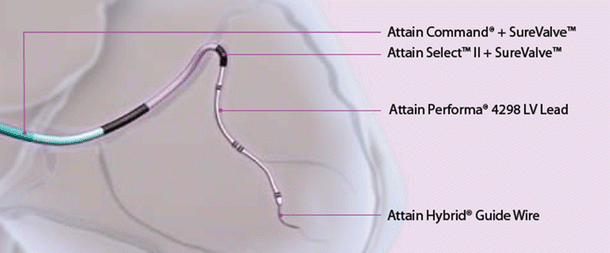

Fig. 31.2
Retrograde coronary sinus venography showing multiple coronary venous branches. Modified from Merkely et al. [166], available under a Creative Commons Attribution license. http://creativecommons.org/license/by/3.0

Fig. 31.3
Illustration of Attain Command® coronary sinus cannulation catheter , Attain Select™ II sub-selection catheter and Attain Performa® LV lead with guidewire. Courtesy of Medtronic plc
31.4.1 Left Ventricular Leads
Currently, a variety of lead body shapes and electrode configurations are available for LV leads. The majority of LV leads employ passive fixation, meaning the lead body shape primarily holds the lead in place. Typical shapes include compound cants, sigmoidal S-shapes, spirals, and straight leads with tines. Since dislodgement remains a concern for all lead systems, LV leads with active fixation components have also been developed. For example, the Attain Starfix® (Model 4195, Medtronic plc) lead was developed specifically to address dislodgement by incorporating deployable lobes on the lead body (Fig. 31.4) [45]. The lobes are deployed by using a tool to push the outer lead body tubing distally and forcing the lobes to expand. More recently, the Attain Stability® (Model 20066, Medtronic plc) lead has been developed with a side helix for fixation, and it has demonstrated acceptable performance through 12 months in humans (Fig. 31.5) [46]. This lead was designed to be positioned in a wider range of vein sizes with enhanced extractability if required.
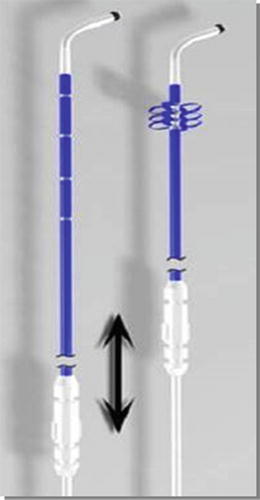


Fig. 31.4
Illustration of Attain StarFix® Model 4195 lead design with deployable lobes for fixation. Courtesy of Medtronic plc

Fig. 31.5
Illustration of Attain Stability® Model 20066 lead design with co-radial helix for fixation. Courtesy of Medtronic plc
A key characteristic of LV leads is the polarity or number of electrodes. Options include unipolar, bipolar, and quadripolar leads (Fig. 31.6). Unipolar leads are still commercially available but are less attractive due to limited options for programming LV stimulation. Bipolar leads are more common and allow three to six stimulation vectors, depending on the capabilities of the pulse generator. More recently, quadripolar LV leads have been developed to further increase the number of pacing vectors, to help manage problems such as high pacing thresholds, PNS, and/or dislodgement [47]. In contrast to unipolar and bipolar leads, quadripolar leads have a newer connector pin which conforms to the ISO 27186:2010 (E) four-pole connector standard (Fig. 31.7). This standard was developed to ensure interchangeability of leads and devices between manufacturers.
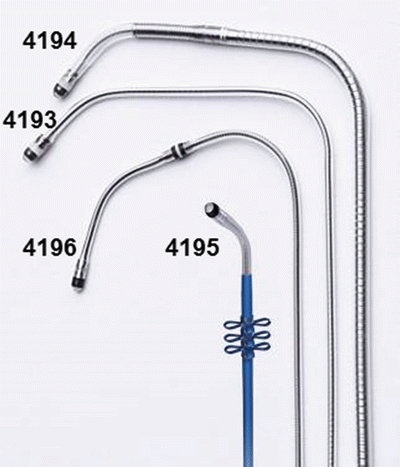
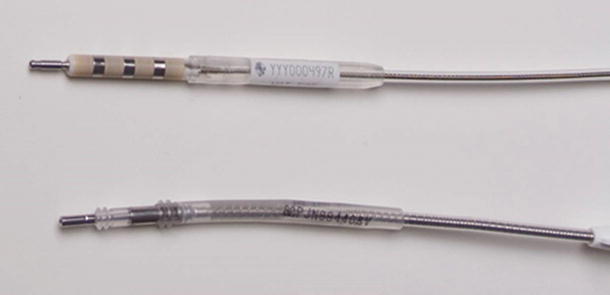

Fig. 31.6
Example of unipolar (4193 & 4195) and bipolar (4194 & 4196) LV leads . Courtesy of Medtronic Inc.

Fig. 31.7
Examples of an IS4 lead connector (top) and IS1 connector (bottom). Courtesy of Medtronic Inc.
31.4.2 Complications Associated with CRT
Complications related to CRT can be divided into either acute or chronic events. Acute complications during implant include: (1) CS dissection; (2) high pacing thresholds; (3) dislodgement; and (4) stimulation of the phrenic nerve. Coronary sinus dissection occurs when the venous wall is damaged, and this is often visualized after injection of contrast. Common causes for this include the advancement of the cannulation catheter, guidewire, lead, or other implant tools into difficult anatomies. Though dissection rarely requires additional treatment intervention, the trauma may be extensive enough to prevent LV lead implantation or it may be severe enough to require pericardiocentesis to prevent tamponade. Left ventricular lead dislodgement may occur during removal of implant tools or it may be due to unstable implant positions. PNS may sometimes occur along the entire length of the target vein. Therefore, dislodgement, high thresholds or inability to capture, and PNS may cause implant failure in some cases.
Complications related to CRT have declined considerably since the therapy was introduced. For example, in one report, implant success rates by French investigators improved from 60 % without dedicated tools in the early years to 98 % by the end of the 1990s; this was accompanied by a reduction in LV lead dislodgements from 30 to 12 % during the same time period [48]. Other studies have demonstrated a learning curve for CRT, with greater implant success and lower complication rates occurring as implanting center and operator experience increases [48, 49]. Today, implant success rates of greater than 95 % are not uncommon with transvenous CRT systems, especially when repeated implant attempts are considered [50–52]. Yet, it should be noted that the etiology of CHF may also impact LV lead implant success. For example, Macias et al. found that ICM was the only independent predictor of a failed LV lead implant at a preferred empirical lateral site [52]. This may be due to lack of veins secondary to scar formation in ICM, since 50 % of ICM patients with a failed lateral implant had no target vein in this region; note that the inability to deliver the lead to the target vein was the most common reason for failed implants in NICM patients. Procedure-related complication rates have reduced over time, from 24 % of patients in the MIRACLE, MIRACLE ICD, and Insync III studies [49] to approximately 16 % in the CARE-HF study [50]. The need for LV lead reintervention after implant varies from 5 to 10 % in studies with up to 1-year follow-up [49, 51, 53]. The most frequent cause of reintervention is dislodgement in approximately 7–10 % of patients; however intractable PNS, high capture thresholds, and infections have also been noted. To date, data on coronary vein thrombosis and implant success rates after LV lead revision are relatively limited. Yet, Borleffs et al. reported an 86 % first success LV lead revision rate at a median of 85 days, and the same branch as the original implant could be used in 57 % of the cases [51]. Additionally, Biffi et al. reported that the original branch could only be used in 33 % of patients with the majority of LV lead revisions occurring less than 6 months post-implant [53].
Clinically relevant PNS is observed in approximately 20 % of patients when the LV lead is placed in a lateral or posterolateral position [54, 55]. The rate of PNS varies considerably by anatomical zone of the LV lead implant. A retrospective analysis of over 1000 patients found that a lateral lead position was associated with greater than four times the risk of PNS than an anterior position, and the apical position was associated with greater than six times the risk [53]. Pacing systems with multiple programmable cathode and anode combinations provide an effective means to avoid high pacing threshold and PNS [54, 55]. Bipolar pacing from a closely spaced electrode, recently incorporated into a quadripolar lead design (Fig. 31.8), has also been demonstrated to avoid PNS by reducing the size of the stimulating electrical fields. The resulting increases in PNS thresholds with minimal change in LV pacing thresholds thus increases the safety margin, and has been demonstrated in both preclinical and human studies [56, 57]. For more specific information about cardiac venous anatomy, see Chap. 8.


Fig. 31.8
Attain Performa® quadripolar LV lead family. Courtesy of Medtronic plc
31.5 Clinical Trials in CRT
31.5.1 Moderate-to-Severe CHF
Multicenter clinical studies of CRT began in the latter half of the 1990s. MUSTIC was a multicenter study which enrolled 67 patients with QRS durations ≥150 ms, LVEFs <35 %, and NYHA class III while receiving optimal medical therapy for at least 1 month [58]. The primary endpoint of this single-blinded, randomized, crossover study was a 6-minute hall walk distance (6MWD) . Secondary endpoints included quality of life (QoL), peak oxygen consumption, CHF hospitalization, and mortality. Patients underwent a period of inactive and active pacing for 3 months each. Distance walked during the active phase was 23 % longer than during the inactive phase (p < 0.001). Quality of life and peak oxygen consumption significantly improved during pacing, and CHF hospitalizations were reduced. PATH-CHF was a single-blinded, randomized, crossover study that enrolled 42 patients. Patients were in NYHA classes III or IV, with QRS durations >120 ms and sinus rhythms; the use of chronic univentricular or BiV pacing configuration was determined by invasive hemodynamic optimization, using aortic pulse pressure and LV dP/dt max [30]. Notably, in patients with BiV therapy, LV end-systolic and end-diastolic volumes decreased, NYHA class improved, and LVEF increased from 22 ± 7 % to 26 ± 9 % (p = 0.03) [59]. Around the same time period, larger randomized, double-blinded studies were initiated to definitively demonstrate the effectiveness of CRT. The Multicenter InSync Randomized Clinical Evaluation (MIRACLE) study enrolled patients with LVEF ≤35 %, QRS duration ≥130 ms, and individuals with moderate-to-severe CHF (NYHA class III or IV) [8]. Patients were randomized to CRT or control (no pacing therapy) for 6 months. Patients receiving CRT had significantly improved 6MWD , QoL, time during treadmill exercise, and LVEF. CRT also reduced LV end-diastolic dimensions and mitral regurgitation. More patients were classified as improved (67 % vs. 39 %) by Packer’s clinical composite score (CCS) and fewer patients were worsened (16 % vs. 27 %) than in the control group (p < 0.001). Further, CHF-related hospitalizations, as well as a combined endpoint of death or CHF hospitalizations, were also reduced in the CRT group. This study led to FDA approval of CRT pacemaker (CRT-P) therapy in August 2001.
The next progression in the use of this therapy was the initiation of trials with combined CRT and defibrillator therapy (CRT-D); these were initiated to determine the additional potential benefits of CRT in patients indicated for ICD . The double-blinded CONTACT CD approval study randomized 490 patients in NYHA classes II–IV, with LVEF ≤35 % and QRS duration ≥120 ms, to either CRT-D or ICD therapy alone for up to 6 months [60]. The primary endpoint was a composite of all-cause mortality, CHF hospitalization, and occurrence of VT/VF requiring ICD therapy. Secondary endpoints included peak levels of VO2, 6MWD, relative NYHA class, QoL, and echocardiographic changes. There was a nonsignificant 15 % reduction in the primary endpoint of the CRT group (p = 0.35). In some patients the NYHA class improved prior to CRT because optimal medical therapy was instituted for 1 month prior to randomization. In the group with NYHA class I–II, there were no significant improvements in any secondary endpoint. In contrast, there were significant improvements in peak VO2, 6MWD , NYHA class, and QoL in patients in NYHA classes III–IV. Based on this study, CRT-D therapy was approved by the FDA in February 2002 for patients with moderate-to-severe CHF (NYHA classes III–IV), LVEF ≤35 %, and QRS duration ≥120 ms.
Subsequently, clinical trials with longer study durations demonstrated the benefits of CRT on mortality. The Comparison of Medical Therapy, Pacing, and Defibrillation in CHF (COMPANION) trial randomized 1520 patients in NYHA classes III–IV, LVEF ≤35 % and QRS duration ≥120 ms into three groups: optimal medical therapy, CRT-P, or CRT-D [10]. The primary endpoint was time to all-cause death or hospitalization, with a secondary endpoint of all-cause death. The primary endpoint was reduced in both the CRT-P (HR = 0.81, p = 0.014) and the CRT-D groups (HR = 0.80, p = 0.01) with 12 months follow-up. The risk of death or hospitalization due to CHF was reduced by 34 % with CRT-P (p < 0.002) and 40 % with CRT-D (p < 0.001). CRT-D reduced the risk of all-cause mortality by 36 % (p = 0.003), although the 24 % reduction with CRT-P did not reach significance (p = 0.06). Next, the Cardiac Resynchronization-Heart Failure (CARE-HF) study demonstrated that CRT alone (CRT-P) reduced the risk of death beyond the benefits of optimal medical therapy [11]. In this trial, 813 patients in NYHA classes III–IV, LVEF ≤35 %, and with QRS duration ≥120 ms were randomized into optimal medical therapy or CRT-P. Patients with QRS intervals between 120 and 149 ms were required to meet 2 of 3 mechanical dyssynchrony criteria for inclusion. The primary endpoints were time to all-cause death or unplanned hospitalization associated with a major cardiovascular event. With a mean follow-up of 29.4 months, 39 % of patients with CRT reached the primary endpoint vs. 55 % of patients receiving only optimal medical therapy (HR = 0.63, p < 0.001). For the principal secondary endpoint of death from any cause, the rates were 20 % in the CRT group vs. 30 % for those patients receiving optimal medical therapy (HR = 0.64, p < 0.002). It was also noted in this trial that CRT improved interventricular mechanical delay, LV ESV, mitral regurgitation, LVEF, and QoL, which was in agreement with previous studies.
31.5.2 Mild CHF
The next major focus of clinical trials and the expansion for indications for CRT was the treatment of patients with milder CHF. The Resynchronization Reverses Remodeling in Systolic Left Ventricular Dysfunction (REVERSE) trial was a double-blinded, randomized study of patients in NYHA classes I–II, QRS ≥120 ms, and LVEF ≤40 % [61]. In this trial, 610 patients were implanted and randomized to CRT, on or off. The primary endpoint was a CHF CCS, and the prospectively powered secondary endpoint was LV ESVi. There were no significant differences in the percentage of patients categorized as “worsened” between CRT on and off over 12 months of follow-up (16 % worsened with CRT on vs. 21 % with CRT off, p = 0.10). However, patients programmed to CRT elicited significant improvements in LV ESVi and had longer time to first CHF hospitalization (HR = 0.47, p = 0.03). The larger Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy (MADIT-CRT) trial randomized 1820 patients in NYHA classes I–II, with QRS duration ≥130 ms and LVEF ≤30 % in a 3:2 ratio to CRT-D or ICD alone [62]. The primary endpoint was a composite of all-cause death or nonfatal CHF events. This primary endpoint was reached in 17.2 % of patients implanted with CRT-D vs. 25.3 % in the ICD group, with an average follow-up of 2.4 years (HR = 0.66, p = 0.001). This was primarily driven by a 41 % reduction in risk of CHF events with CRT, which was greater in a prespecified group of patients with QRS ≥150 ms. The annual mortality rates in each group were similar at approximately 3 %. Improvements in LV volumes and LVEF, from baseline to 1 year, were also significantly greater in the CRT group.
The double-blinded, Resynchronization–Defibrillation for Ambulatory Heart Failure Trial (RAFT) extended these results to patients in sinus rhythm or with rate-controlled permanent atrial fibrillation (AF) or atrial flutter [63]. Altogether, 1798 patients in NYHA classes II–III, QRS duration ≥120 ms, and LVEF ≤30 % were randomized to either CRT-D or ICD. The original protocol was later revised to enroll only patients in NYHA class II, resulting in a total of 20 % of patients in NYHA class III. The primary endpoint of all-cause death or CHF hospitalization was lower in the CRT-D group (33.2 %) than in the ICD group (40.3 %). Time to first primary endpoint was significantly prolonged with CRT-D (HR = 0.75, p < 0.001). Both death and CHF hospitalizations were significantly lower in the CRT-D group, though this was associated with approximately twice the number of adverse events at 30 days postimplant.
31.5.3 Mechanical Dyssynchrony, Narrow QRS Duration, and AV Block
The role of mechanical dyssynchrony for improving patient selection for CRT remains controversial. The multicenter, nonrandomized Predictors of Response to CRT (PROSPECT) study evaluated the ability of 12 echocardiographic indices of dyssynchrony to predict CRT responses at 6 months [64]. A positive response was defined as an improved CCS, with at least a 15 % reduction in LV ESV. These indices provided only modest sensitivity and specificity, and the investigators reported large variability in quantification of dyssynchrony. Mechanical dyssynchrony has also been used to select CRT candidates with a narrow QRS duration ≤120 ms, with limited success in randomized multicenter studies. The earliest of these studies, the Cardiac Resynchronization Therapy in Patients with Heart Failure and Narrow QRS (RethinQ) study, was a double-blinded, randomized study of patients in NYHA class III, LVEF ≤35 %, and QRS duration ≤120 ms with evidence of mechanical dyssynchrony [65]. Tissue Doppler imaging (TDI) or M-mode delay between opposite cardiac wall segments were used to determine the presence of dyssynchrony. The primary endpoint of improved peak VO2 at 6 months was not significantly improved with CRT. There were no significant differences in QoL, 6MWD, or cardiac structure and function between CRT-on and CRT-off, although more patients experienced significant improvement in NYHA class with CRT (54 % vs. 26 %, p = 0.006). It should be noted that these findings were in contrast to smaller, single center studies reporting benefits in patients with narrow QRS duration [66–68]. The largest study in this population, the Echocardiography Guided Cardiac Resynchronization Therapy (EchoCRT) study , randomized 809 patients implanted with CRT-D to CRT-D or ICD therapies only [69]. Patients in NYHA classes III–IV, LVEF ≤35 %, and QRS duration ≤130 ms with evidence of mechanical dyssynchrony (defined by TDI or speckle tracking radial strain) were enrolled. The primary efficacy endpoint was all-cause death or CHF hospitalization. This study was stopped for futility, with a potential for harm based on recommendations from the data and safety monitoring board in 2013. The differences in the primary endpoint were not different between the CRT and ICD groups (28.7 % vs. 25.2 %, HR = 1.2, p = 0.15). Yet, there was a significantly higher rate of death in the CRT group (11.1 % vs. 6.4 %, HR = 1.81, p = 0.02), with more deaths due to cardiovascular causes, and there were no differences at 6 months in NYHA class or QoL. This study further reinforced the importance of QRS duration over mechanical dyssynchrony as the more important determinant of CRT responses.
One potential group of patients with CHF and narrow QRS durations that may benefit from CRT are those with AV block who then require a high percentage of ventricular pacing. The prospective, double-blinded, randomized Biventricular versus Right Ventricular Pacing in CHF Patients with AV Block (BLOCK HF) study enrolled patients in NYHA classes I–III, LVEF ≤50 %, and a standard class I or IIa indication for pacing due to high-degree AV block [70]. In this trial, 691 patients were implanted with CRT-D or CRT-P, randomized to CRT or RV pacing, and followed for an average of 37 months. The primary endpoint was time to event, which included death from any cause, urgent care visits for CHF requiring IV therapy, or ≥15 % increase in LV ESV index. It was shown that 55.6 % of patients in the RV pacing group met the primary endpoint, significantly more than the 45.8 % patients in the CRT group (HR = 0.74). The secondary endpoints of death from any cause or urgent care CHF visits, death or CHF hospitalization, and CHF hospitalization were also significantly reduced with CRT. This trial led to an expanded indication in April 2014 for use of CRT devices in patients in NYHA classes I–III with AV block and LVEF ≤50 % who are expected to require a high percentage of ventricular pacing. The Biventricular Pacing for AV Block to Prevent Cardiac Desynchronization (BioPace) study is an ongoing trial in a similar patient cohort. Approximately 1900 patients with a class I indications for permanent ventricular pacing and high likelihood of ventricular pacing ≥66 %, without restriction on LVEF, were randomized and implanted with a CRT or RV pacing systems [71]. The primary endpoints include all-cause mortality and time to first CHF hospitalization.
31.6 Factors Influencing CRT Responses
31.6.1 QRS Duration, Morphology, and QRS to LV EGM Onset (QLV)
The relative QRS duration provides powerful prognostic value for patients with CHF and is a primary indicator of eligibility for CRT. A cut-off of 120 ms for QRS duration was established in the initial guidelines, based on randomized trials of CRT, although smaller studies suggested that patients with a QRS duration <150 ms had limited benefit from CRT [30]. Evidence obtained from REVERSE, MADIT-CRT, and RAFT trials, as well as recent meta-analyses, has concluded that patients with QRS durations of at least 150 ms derive greater benefit from CRT. Patients with QRS durations <140 ms did not experience significant reverse remodeling in the REVERSE trial [72]. Analysis of the prespecified subgroup of patients with QRS durations <150 ms did not elicit significant reductions in death or CHF in the MADIT-CRT [62] or RAFT [63] trials. Similarly, meta-analyses of COMPANION, CARE-HF, RAFT, MADIT-CRT, and REVERSE concluded that CRT did not reduce clinical events in patients with QRS durations <150 ms [73]. Analysis of almost 15,000 patients in the Medicare ICD registry, implanted between 2005 and 2006, identified that patients with QRS durations >150 ms had significantly lower mortality and combined mortality or CHF hospitalizations at both 1 and 3 years after CRT [74]. A prospective 10-year observational registry study demonstrated that mortality after CRT benefits were similar between patients with QRS durations of 120–149 ms and those of 150–199 ms, with significantly increased mortality in patients with QRS durations of 200 ms and greater [75]. This finding is not inconsistent with studies concluding that patients with QRS durations of 150 ms and greater benefit more with CRT, since CRT may offset the greater mortality rate associated with increasing QRS durations.
Recent studies have also concluded that patients with LBBB are more likely to respond to CRT than those with right bundle branch block (RBBB) or nonspecific interventricular conduction delays (IVCDs). This is somewhat intuitive, since correction of delayed lateral wall contraction with early activation of the septum in LBBB is the hallmark of CRT. An LBBB morphology was the only baseline ECG characteristic that was significantly associated with improved clinical composite score (CCS) and reverse remodeling in a sub-study of PROSPECT [76]. CRT reduced the risk of CHF hospitalization or death by 53 %, relative to ICD in patients with LBBB in MADIT-CRT, with no significant reduction in patients with non-LBBB morphology [77]. It should be noted that similar findings were found in the RAFT trial [63]. Meta-analyses of COMPANION, CARE-HF, MADIT-CRT, and RAFT data have demonstrated greater benefits in reduction of mortality and morbidity in patients with LBBB vs. IVCD or RBBB [78]. Analyses of the Medicare ICD registry also revealed that patients with RBBB had significantly greater mortality than those with LBBB after adjusting for baseline covariates [74]. Recently, Adelstein et al. also reported that patients with RBBB elicited less reverse remodeling, lower improvement in NYHA at 6 months, and had lower survival and fewer transplants or LVADs than patients with LBBB [79].
Left ventricular lead placement at a site of latest electrical delay, measured by local EGM, is also associated with an increased probability of a positive response to CRT (Fig. 31.9). Some of the earliest evidence supporting this concept was demonstrated in the PATH-CHF-II study, where the difference in LV conduction delay between the free wall and anterior coronary veins was correlated with the difference in improved LV dP/dt max while pacing within each vein [41]. Another study found that both RV to LV conduction and QLV (i.e., QRS onset to LV EGM) correlated with improved LV dP/dt max during optimized BiV pacing; yet no clear cut-off was identified to predict acute hemodynamic response [80]. Normalizing the QLV by QRS duration, termed LV lead electrical delay (LVLED), was also shown to correlate with Doppler-derived dP/dt values, and an LVLED greater than or equal to 50 % was associated with significantly greater reductions in all-cause death or CHF hospitalization at 12 months follow-up in patients with ICM or NICM [81]. The same group of investigators later showed that LVLED was similarly associated with improved outcomes in patients with apically placed LV leads. An LVLED of at least 50 % with an apical LV lead placement was associated with greater freedom of all-cause deaths, CHF hospitalizations, and need for transplant at 2 years (81 % vs. 30 %, p = 0.007), as well as greater reductions in LV ESV and increased LVEF [82]. Similarly, Gold et al. also found that longer QLVs were associated with improvements in reverse remodeling and QoL in a subanalysis of SMART-AV [83]. A QLV of 120–195 ms was associated with 3.2 times greater odds of reverse remodeling compared to a QLV <70 ms, via multivariate analysis. However, the ability of QLV-guided LV lead placement to improve outcomes after CRT remains to be tested in prospective, randomized studies.
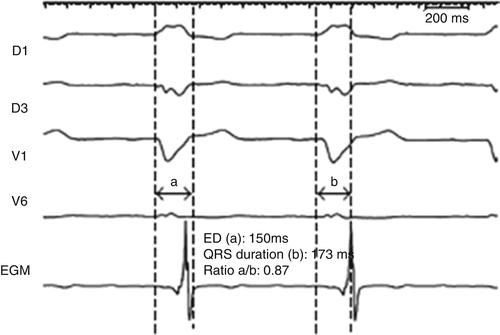

Fig. 31.9
QLV electrical delay (a) and QRS duration measurement . Modified from Fatemi et al. [167], available under a Creative Commons Attribution license. http://creativecommons.org/license/by/2.0
31.6.2 LV Lead Position, Scar, and Mechanical Dyssynchrony
Left ventricular lead position has been recognized as an important determinant for response to CRT since the initial development of this therapy. Initially, acute studies demonstrated greater improvements in hemodynamics during LV free wall pacing than during anterior stimulation, thus providing the basis of empirical targeting of lateral or posterolateral veins [41]. In this series of studies, pacing anterior locations actually decreased LV dP/dt max and pulse pressures below baseline levels in approximately one-third of patients. However, a lateral position cannot be assumed to provide the maximal benefit for all patients. For example, Dekker et al. demonstrated that even though pacing the mid-lateral or basal LV segments corresponded to the best hemodynamic function during surgical epicardial mapping in the majority of patients, these regions corresponded to the worst function in other patients [42]. Expanding on these findings, Gold et al. tested multiple pacing locations within a lateral or anterior vein to determine the impact of activating different sites within a vein [84]. They observed large individual variations in the hemodynamic responses between apical and basal regions, with no significant differences between both regions on average. Additional acute studies investigating the benefits of LV endocardial pacing have confirmed that the location of the optimal pacing site varies significantly between patients, supporting a strategy of individualized LV lead placement to maximize the benefit of CRT [85, 86].
Retrospective analyses of large clinical studies have provided additional insights as to the role of anatomical LV lead positions on chronic outcomes after CRT. Multiple studies have concluded that a more apical LV lead position carries a negative prognosis, while the role of circumferential lead position is less certain. An apical lead position was associated with significantly increased risk for CHF hospitalizations or death after adjusting for clinical covariates in MADIT-CRT [43]. Similar impacts of apical LV lead positions on CHF hospitalization and death were demonstrated in other retrospective single center and multicenter studies [87–89]. Apical lead placement has also been associated with lower levels of subsequent reverse remodeling and a smaller improvement in the patient’s assessed NYHA class [87, 88]. Anterior LV lead positions have traditionally been avoided, but data on the impact of circumferential position on chronic outcomes after CRT are conflicting. For example, in one assessment there were no significant differences in outcome between lateral, anterior, and posterior lead positions in MADIT-CRT [43]. Changes in 6MWD , QoL, and percentage of patients with improved NYHA class were similar between lead positions in COMPANION [90]. Similarly, there were no differences in all-cause mortality or CHF hospitalizations, though risk of all-cause mortality or all-cause hospitalizations was not significantly reduced in patients with posterior leads. In contrast, two retrospective analyses found that lateral lead positions were associated with lower incidence of death or first hospitalizations [87] and two to three times less risk for a non-response, death, or necessary transplant [91].
31.6.2.1 Role of Baseline Mechanical Dyssynchrony, Scar, and Implications for LV Lead Position
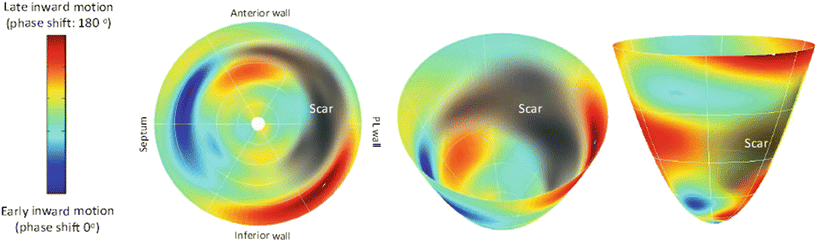
Corrections of AV, interventricular , and intraventricular dyssynchrony are believed to be the major mechanisms of action for improved cardiac function with CRT. Numerous studies have concluded that the presence of baseline dyssynchrony improves the likelihood of a patient’s CRT response. For example, an interventricular mechanical delay of at least 40 ms was an independent predictor of response in a sub-analysis of the CARE-HF trial [92]. Though the PROSPECT study reported a relatively weak ability of echocardiographic measures of dyssynchrony to predict outcomes after CRT, subsequent analyses concluded that patients who experienced improvement in both CCS and reverse remodeling had greater baseline dyssynchrony [93]. Furthermore, a recent study utilizing speckle tracking radial strain showed that a lack of baseline radial strain was associated with worse freedom from death, transplant, or LVAD [94]. Cardiac magnetic resonance imaging (MRI) and single-photon emission computed tomography (SPECT) are also useful for the quantification of mechanical dyssynchrony, tissue viability and scar (Fig. 31.10) [95, 96].
 < div class='tao-gold-member'>
< div class='tao-gold-member'>





Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


