Fig. 18.1
Percentage of patients with each indication for device implantation. All are new implants since 2000. Cong Block = nonsurgically related heart block; Surg Block = surgically related heart block; Surg SND = surgically related sinus node dysfunction; Cong SND = nonsurgically related sinus node dysfunction; Tachy Control = device placed for control of tachyarrhythmia
Pacemaker implantation is mandatory for all patients with surgically induced and permanent (≥7 days) complete heart block. Escape heart rate mechanisms are slow and tend to be unstable with a high incidence of sudden asystole. No patient is discharged following cardiac surgery without pacemaker implantation unless there is 100 % intact atrioventricular conduction. While later recovery of conduction can occur, it is unlikely to have complete return of conduction. Pacemaker placement is usually performed 7–14 days post-op. For patients with return of AV conduction, they must have documented 100 % AV conduction by 24-h Holter monitoring to avert pacemaker implantation.
For patients with late onset complete heart block following cardiac surgery, pacemaker placement is also recommended without requiring associated symptomatology. For these patients, the heart block is usually not persistent but intermittent. The risk of sudden syncopal episodes is considered to be elevated and thus pacemaker implantation is recommended. In addition, late resolution of postsurgical heart block (beyond 7–10 days) appears to increase the development of late onset complete heart block, hence the recommendation for pacemaker implant and follow-up.
The second largest and the most rapidly expanding group of patients that require device implantation are those with sinus node dysfunction. While most of these patients have undergone previous cardiac surgery, some have no other cardiac disease. Documented low atrial rates with or without junctional escape rhythms, along with symptoms of intermittent or chronic low cardiac output, tend to worsen with time and warrant pacemaker implantation. The younger patients with sinus node dysfunction following cardiac surgery may not require pacing, whereas patients in their late teenage and early adult years often require device implantation. Patients who have undergone extensive atrial surgery such as the Fontan procedure or the atrial switch repair of transposition of the great arteries are not only at high risk for developing sinus node disease but also for atrial tachycardias, that may in part be unmasked by low atrial rates. Control of atrial tachycardias can be greatly aided by maintenance of a minimal atrial rate of 70–80 bpm.
Tachyarrhythmias
Children who require treatment with antiarrhythmic agents for control of tachyarrhythmias that result in resting bradycardia may often benefit from pacemaker implantation. However, these children are often candidates for implantation of an anti-tachycardia device with bradycardia pacing support. Children with hypertrophic obstructive cardiomyopathy and significant left ventricular outflow tract gradients have been shown in some studies to benefit from dual-chamber pacing to asynchronously activate the ventricles, potentially relieving intracavitary obstruction. However, this is a very controversial recommendation and has been rarely employed in our practices. If they become symptomatic (with syncope) or have complex ventricular ectopy, these children may require a combination of bradycardia pacing and an implantable defibrillator.
Indications for placement of an anti-tachycardia device in children are imprecise. Anti-tachycardia devices can be divided into two types. The first type is capable of delivering anti-tachycardia pacing and defibrillator shocks and the second type can perform only anti-tachycardia pacing. A patient who has been resuscitated from a sudden cardiac death event or has been pre-syncopal with documented ventricular tachycardia requires placement of an Implantable cardioverter-defibrillator (ICD)—i.e., secondary prevention. However, primary prevention for those children that are felt to be at risk of sudden cardiac arrest has become more prevalent, paralleling the increase in genetic testing. Children who have cardiac conditions which have a high incidence of ventricular arrhythmia, such as tetralogy of Fallot or cardiomyopathy, and who also have had documented significant ventricular ectopy are increasingly undergoing ICD placement. A long QRS duration in a patient following tetralogy of Fallot repair has also been reported as a risk factor for ventricular arrhythmia and an indication for ICD placement. However, this proposal is very controversial and is currently not used in our practices as a sole criterion for ICD placement.
Patients with atrial flutter are also being considered in specific situations for cardiac pacing. By keeping the base atrial rate above a predefined number, usually 70–85 bpm, and/or preferentially pacing slightly above the sinus rate, the burden of atrial flutter may be decreased. In addition, when this practice is coupled with anti-tachycardia pacing, control of atrial flutter may be significantly improved. The increased use of an anti-tachycardia dual-chamber rate responsive pacemaker may lead to an increase in this therapy for atrial tachyarrhythmias in children with sinus node dysfunction. Pacing algorithms for prevention of atrial flutter have been proposed but to date have not been shown to be universally effective.
Heart Failure
For patients with heart failure, cardiac resynchronization is based on the concept that the difference in activation between the right and left ventricles due to intraventricular conduction disturbances, as in left and right bundle branch block or ventricular pacing, leads to decreased ventricular performance. Pacing both ventricles in a coupled manner may lead to more normal biventricular excitation (i.e., resynchronization) and thus improve ventricular function. This technology is an established technique to improve cardiac function and quality of life in adults with significant congestive heart failure and ventricular dysynchrony manifested by a decreased left ventricular ejection fraction and a wide QRS complex. The treatment of children with biventricular pacing for improvement of ventricular performance and increased cardiac output is evolving and becoming more widely employed. A number of short- and mid-term results from single- and multi-center studies indicate that cardiac resynchronization is feasible and effective in children and young adults with congenital heart disease and heart failure. However, the indications for biventricular pacing and a candidate population of young patients have not been fully defined.
Implantation Choices
When it has been decided that a child will benefit from cardiac pacing, several decisions must be reached to provide the most optimal therapy. The first decision is whether or not epicardial or endocardial electrode implantation is optimal. The second decision is whether dual-chamber or single-chamber pacing is in the child’s best interest. Once these decisions have been reached, attention can be turned towards the choice of appropriate electrodes and, finally, the implantable device itself. One must consider not only what is in the child’s immediate best interest and clinical situation, but also how the clinical state may change over time. Such decisions must take into consideration the potential for future adverse events that might be avoided or better treated with more appropriate initial choices of implantation route and devices.
Epicardial Versus Endocardial Placement
The first decision to be made following the recommendation for pacemaker implantation is whether the electrodes are to be implanted on the epicardial or endocardial surface. This decision clearly depends on the availability of venous access to the atrium and the pulmonary ventricle, as well as patient size and the presence or absence of any other conditions considered as contraindications for endocardial electrode placement (e.g., intracardiac right to left shunt). Patients in whom there is no venous route to the ventricle and atrium must undergo an epicardial implant. Often superior vena caval obstruction can often be opened by balloon dilatation and stent placement to permit passage of endocardial pacemaker leads. While there have been isolated reports of inferior vena caval passage with introduction of the electrodes in the femoral region and generator implantation in the abdomen, this is not considered optimal, due to the problems encountered with growth and the fact that such implantation may prohibit any further use of the femoral vessels for central venous access.
Patients in whom there is no pulmonary ventricle (e.g., patients with single ventricles following the Fontan procedure) are in general not candidates for transvenous pacing; in rare circumstances, transvenous ventricular pacing can be performed via the coronary sinus. Implantation of a transvenous atrial pacemaker lead in the Fontan circuit has been reported but is controversial. With the low flow state and the potential for clot formation, the possibility of right to left shunting through either a fenestration in the Fontan baffle or baffle leaks, any thrombi have the potential for systemic embolization. Another problem is the risk of pulmonary embolization and if the thrombus is large, dire consequences. In our practices such an approach is seldom used, and when used, long-term anticoagulation therapy is recommended.
There have been reports of pacemaker electrode placement within the systemic ventricle, but this approach can be associated with embolization and CNS injury. Even patients who have been maintained on Coumadin at adequate levels may still experience micro-emboli from the pacing lead. For this reason, lead placement in the systemic ventricle is not performed in our practices.
Patient size has long been debated as to its effects on the choice of electrode route. While reports of infants undergoing transvenous implantation abound, the long-term sequelae have not been well studied. With the recently developed smaller caliber transvenous leads, great vein obstruction becomes less of an issue. However, the development of significant SVC or innominate vein obstruction in a young child would severely jeopardize future electrode placement. Also the effects on tricuspid valve function in the presence of multiple electrode leads are also a concern. While electrode extraction is a possible solution, it carries a significant risk. At the current time in our practices, the transvenous approach is not used for children less than 15 kg in weight for a single lead and less than 25 kg for those requiring a dual lead system. An obvious advantage of the epicardial approach is the increase in the potential sites for pacing in an individual who will require lifelong pacing and multiple revisions as pacing leads fail. As electrodes become smaller and electrode extraction becomes safer, this consideration may change.
Contraindications to endocardial electrode placement are the presence of right to left intracardiac shunting, a hypercoagulability state, and pulmonary vascular obstructive disease. Transvenous leads must be avoided in patients in whom any small emboli to the lungs would further increase pulmonary resistance.
Bipolar Versus Unipolar Electrode Usage
The next issue that must be decided is whether one will utilize bipolar or unipolar electrodes. Initially, epicardial implantation always required unipolar electrodes, as this was the only type of electrode that was available. The introduction of several types of bipolar electrodes has eliminated this issue. Bipolar implantation, in both transvenous and epicardial settings, has the advantage of creating less extracardiac stimulation and less difficulty with myo-potential extracardiac sensing. When bipolar atrial electrodes are used, there is less difficulty with far-field R-wave over-sensing, which can be a major issue if one is using an anti-tachycardia device (Fig. 18.2). In addition, bipolar electrodes tend to have higher impedance than do unipolar electrodes, thus decreasing current drain for similar output settings with subsequent increased generator longevity. This is a consequence of a smaller surface area in the bipolar pair, as the second electrode in a unipolar system is the device casing (“can”) with a large surface area.
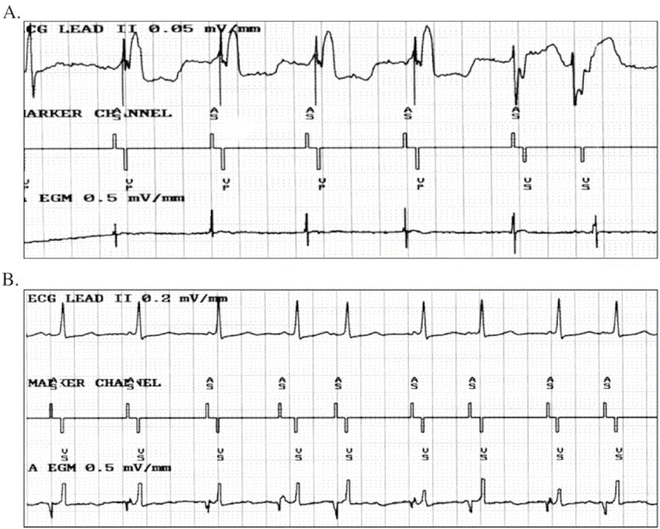

Fig. 18.2
Atrial electrograms from a patient with a bipolar electrode (a) versus a unipolar electrode (b). Top tracing is the body surface EKG; middle tracing is the pacemaker marker channel; third tracing is the atrial electrogram. Note the lack of a far-field R-wave in Panel (a) even with ventricular pacing compared to the large far-field R-wave in panel (b) that exceeds the atrial amplitude
Epicardial Electrodes
Two types of epicardial electrodes are available (Fig. 18.3). The first type, the intramyocardial electrode, penetrates the epicardial surface (Fig. 18.3a–c) and the second type, the epimyocardial electrode (Fig. 18.3d) has a flat plate design and rests on the epicardial surface. Both of these work adequately, but cautions must be observed. Both types of electrodes must be fixed to the epicardial surface with sutures. Failure to suture the electrode to the epicardial surface results in excessive motion of the electrode, which can result in fracture of the electrode or increased fibrosis around the electrode due to excessive irritation. Both events result in loss of pacing.
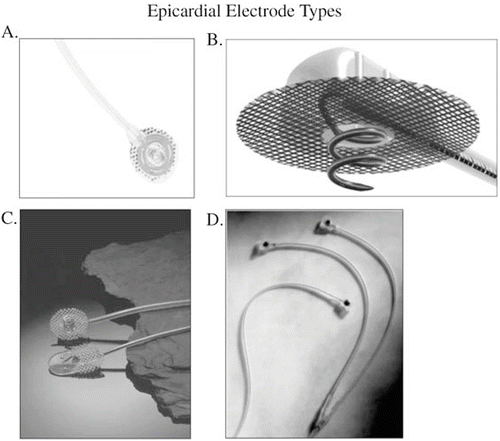

Fig. 18.3
Examples of both bipolar and unipolar epicardial electrodes. (a) Guidant helical bipolar intramyocardial electrode. (b) Medtronic 5071 unipolar helical intramyocardial electrode. (c) Medtronic 4951 intramyocardial barbed electrode (lower electrode) compared with the 5071 electrode (upper electrode). (d) Medtronic 4965 steroid eluting epimyocardial unipolar electrode (left) and the 4968 bipolar steroid eluting epimyocardial electrode (right). (a) [Image provided courtesy of Boston Scientific. © 2015 Boston Scientific Corporation or its affiliates. All rights reserved.] (b–d) [Reproduced with permission of Medtronic, Inc.]
The intramyocardial bipolar electrode has a central helix surrounded by a circular plate that sits on the epicardial surface. This electrode has the advantage of being able to penetrate surface scarring or fat but the ground portion of the electrode that sits on the surface is still prone to being affected by the scarring and epicardial fat. Furthermore, it is not steroid eluting. Care must also be taken with the corkscrew electrodes because only the distal portion of the electrode of both types is active. The electrode should not be placed completely transmural with the tip of the electrode sitting within the blood pool of the ventricular cavity. This results in a low impedance circuit preventing myocardial stimulation. The proximal portion of the electrode has an insulating coating to decrease the surface area and increase the electrode impedance but limits the electrode surface area that is in contact with the myocardium. For this reason, helical electrodes cannot be used in the atrium. Due to these limitations, we have reserved the use of the intramyocardial electrode to those patients in whom the use of the epimyocardial electrode is precluded due to extensive epicardial scarring.
The second type of epicardial electrode is a flat epimyocardial electrode plate that sits on the epicardial surface. It is available in both a unipolar and a bipolar form. The epimyocardial bipolar electrode has two separate heads, each of which must be sutured to the epicardium individually, which increases implant time and requires two separate areas of viable myocardium (Fig. 18.3d). However, its sensing vector can be optimally chosen to avoid far-field R-wave sensing in the atrium and maximize R-wave amplitude in the ventricle. It is also steroid eluting. These electrodes do not penetrate into the myocardium and therefore do not cause as much irritation and subsequent fibrosis as the intramyocardial type of electrodes. In addition, they are steroid eluting, further decreasing the fibrosis around the tip. However, if there is significant scar tissue or epicardial fat present, it can be difficult to find a section of heart muscle that can be stimulated. Thus, surgical implant times may be increased. Yet the improved thresholds and higher impedances make this electrode the epicardial implant of choice in our practice for both the atrium and ventricle.
Endocardial Electrodes
Endocardial electrodes can also be either unipolar or bipolar. Early unipolar electrodes were smaller than their bipolar equivalents and thus were preferred in children. With advances in electrode technology, this size difference has disappeared, leaving almost no advantages to using unipolar transvenous electrodes. The transvenous bipolar electrode has a relatively long rigid tip compared to the unipolar equivalent. In small atrial chambers such as those following the atrial switch repair of transposition of the great arteries, the unipolar electrode may be advantageous. However, only bipolar electrodes can be used with the anti-tachycardia devices.
The major decision to be made for transvenous implantation is between the methods of electrode fixation to the endocardial surface. Electrodes can either lodge in the trabecular recesses held in place by tines (passive fixation) or can penetrate the endocardial surface via a helix that serves not only as the active electrode but also to hold the electrode in place (active fixation). The cross-sectional surface areas of the two electrode types are comparable and the time needed to implant them is similar. For atrial electrodes and ventricular electrodes implanted in the morphologic left ventricle in patients with l-transposition of the great arteries or in patients with d-transposition of the great arteries and the atrial switch operation, active fixation electrodes are much more widely used. When right ventricular pacing at sites other that the apex is needed, active fixation electrodes are required. Active fixation electrodes may also be easier to extract.
In our practices, bipolar electrodes are used for both epicardial and endocardial approaches. The choice between passive and active fixation is more open; while both are available, active fixation types are preferred. Because extracardiac pacing and far-field R-wave sensing is less and both the acute and chronic thresholds are as good as or better than the intramyocardial electrodes, for epicardial implants bipolar epimyocardial electrodes are used. There are times, however, when this electrode type cannot be used due to excessive myocardial scar formation from prior cardiac surgical procedures; intramyocardial electrodes are then required.
In some instances, both transvenous and epicardial leads are used within the same system, a hybrid approach. This is most often employed in cardiac resynchronization therapy when the coronary sinus anatomy is suboptimal for appropriate left ventricular lead placement, when there is no transvenous access for multisite pacing, when necessitated by the cardiac anatomy (single ventricle), or when faced with small patient size.
Dual- Versus Single-Chamber Pacemakers
The next major decision that must be made is whether to use a single- or dual-chamber pacemaker. Pacemakers are classified according to the nomenclature schema devised by the Heart Rhythm Society (HRS) and the British Pacing and Electrophysiology Group (BPEG) (Fig. 18.4a). This scheme has a five-character descriptor for each device. The first column refers to the chamber or chambers being paced, the second to the chamber(s) being sensed, the third to the action taken upon a spontaneous beat occurring, the fourth to the presence or absence of activity sensing, and the fifth to the action taken when a tachycardia occurs. A similar schema has also been proposed for implantable cardioverter-defibrillators (Fig. 18.4b). For the purposes of this discussion, single-chamber pacemakers are defined as those that only have a single lead with no capability of accommodating a second electrode. On the other hand, a dual-chamber unit that has only a single lead with the other port plugged is considered a dual-chamber unit even though it is used in a single-chamber mode, a distinction that will be addressed later.
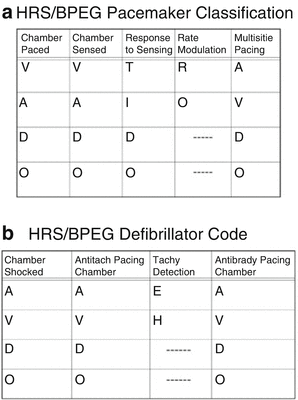

Fig. 18.4
(a) HRS/BPEG schema for pacemaker classification. (b) HRS/BPEG schema for defibrillator classification. See text for details. (a) [Reprinted from Bernstein AD, Daubert J-C, Fletcher RD, et al. The Revised NASPE/BPEG Generic Code for Antibradycardia, Adaptive-Rate, and Multisite Pacing. PACE 2003;25:260–264. With permission from John Wiley & Sons.] (b) [Reprinted from Bernstein AD, Camm J, Fisher JD, et al. The NASPE*/BPEG** Defibrillator Code. PACE 2006;16:1776–1780. With permission from John Wiley & Sons.]
For patients that have intact atrioventricular conduction but require pacing due to sinus node dysfunction, atrial pacing, while technically single-chamber pacing, produces the same advantages as dual-chamber pacing. It is acceptable to utilize single-chamber atrial pacing in those patients who have documented reliable atrioventricular conduction and in whom ventricular pacing is unlikely to be needed in the future. The only exception to this is for patients who require anti-tachycardia devices. These pacemakers, even when programmed in the single chamber mode for atrial bradycardia pacing, require a ventricular electrode for accurate detection of atrial tachyarrhythmias.
Single-chamber ventricular pacing can often be used in the young, small patient with congenital complete heart block and an otherwise structurally normal heart. These patients tend to have good myocardial function and placement of only a ventricular epicardial electrode requires a much smaller surgical procedure. However, whenever there is a question of the adequacy of myocardial function, dual-chamber pacing with preservation of atrioventricular synchrony is preferred. Numerous studies have shown the superiority of dual-chamber versus single-chamber pacing for maintenance of cardiac output in the patient with structural cardiac disease. In addition, heart rate variability is improved when one utilizes the patient’s sinus node to set the heart rate rather than an activity sensor. While activity sensors do provide some heart rate variability in older ambulatory patients, they do not provide as physiologic a heart rate response as the patient’s sinus node does. In the nonambulatory neonate, the sensor is incapable of detecting the type of activity needed to vary the heart rate because activity sensors are not reliable and are of no benefit. Even minute ventilation respiratory sensors are of limited use.
Dual-chamber pacemakers are slightly larger than single-chamber devices due to the need for the pacemaker to accept two electrodes, but usually the difference is not clinically significant. Size differences are usually more a function of battery size rather than connector size. Standard single- and dual-chamber pacemakers can be implanted in the smallest neonate (with epicardial leads). Device longevity is not significantly affected by the added burden of atrial sensing. If atrial pacing is needed, then a dual-chamber device is a better choice.
One caution must be considered. When a dual-chamber pacemaker reaches elective replacement time (ERT), it reverts to VVI pacing irrespective of the programmed mode. If a dual-chamber unit is programmed AAI and there is no functional ventricular electrode, it is possible the device will essentially immediately stop pacing without warning. This must be considered for a dual-chamber device in a patient with a nonfunctional ventricular electrode. Newer devices are addressing this concern and can maintain atrial pacing even when it reaches ERT.
Dual-chamber pacing is preferred in most cases. In our experience since 2001, less than 10 % of implanted pacemakers are single-chamber units (Fig. 18.5). Single-chamber ventricular devices are used in some neonates with congenital complete heart block and normal cardiac structure and function, in patients who rarely require pacing but have a “rescue” device to prevent acute bradycardia secondary to drug therapy or intrinsic electrical system disease, and in the rare situation when an atrial electrode cannot be implanted.
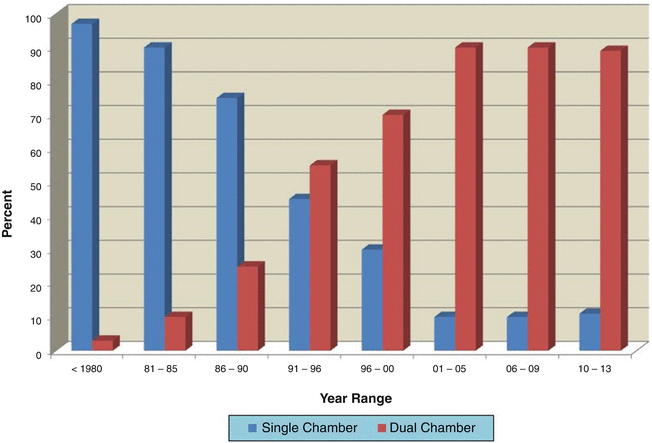

Fig. 18.5
Percentage of patients undergoing dual- versus single-chamber pacemaker implants at the University of Michigan Congenital Heart Center versus the year of implant. Overall about 75 % of implants are dual-chamber devices
Implantable Cardioverter-Defibrillators
The use of an implantable cardioverter-defibrillator (ICD) in children has become an increasingly employed therapy for those with life-threatening arrhythmias. The appropriate selection is similar to that for pacemakers, involving a choice between a dual-chamber or single-chamber device, as well as a transvenous endocardial versus epicardial approach. Similar to pacemakers, a dual-chamber ICD requires an additional electrode, but the size difference of the device is not significant. If the patient’s status calls for dual-chamber pacing, then a dual-chamber ICD is necessary. For patients that do not require pacing except potentially following defibrillation, then single-chamber devices may be acceptable. However, a single-chamber device does not have the ability to discriminate sinus tachycardia from slow ventricular tachycardia or to treat atrial tachyarrhythmias should one develop. If there have been documented atrial tachyarrhythmias in addition to ventricular tachyarrhythmias, a dual-chamber device is strongly preferred.
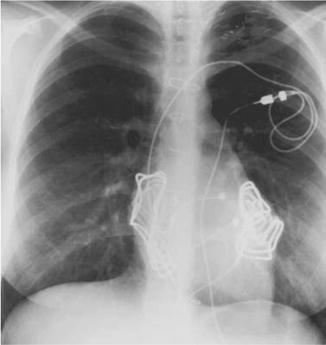
For patients that do not have venous access to the ventricle or are already undergoing a median sternotomy for cardiac surgery, an epicardial approach can be used. Epicardial placement of the defibrillatory patches (Fig. 18.6) can be performed with adequate defibrillatory thresholds (DFTs) obtained; however, bipolar epicardial sensing electrodes are required. In the rare, very small patient, coil electrodes designed for SVC placement can be placed around the heart in the mediastinum to serve as defibrillatory electrodes rather than patch electrodes. It is also possible to place subcutaneous coils in an infrascapular position and the ICD generator in a right upper quadrant abdominal position, creating a defibrillation vector through the heart (Fig. 18.7). While feasible, these hybrid systems are more susceptible to lead failure and should be reserved for those patients in which transvenous access is contraindicated.
 < div class='tao-gold-member'>
< div class='tao-gold-member'>





Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


