Cardiac Pacemakers
Mandeep Bhargava
Bruce L. Wilkoff
History and Overview
The cardiac conduction system possesses the unique capability of generating its own electrical rhythm. The sinoatrial (SA) node, located at the junction of the right atrium and the superior vena cava, possesses the fastest rate of spontaneous depolarization and governs the pace of the heart under normal conditions. After traversing the atrial tissue and then the atrioventricular (AV) node, this impulse reaches the ventricles through the specialized conduction fibers of the His bundle, the right and left bundle branches, and the Purkinje fibers. Disorders of the cardiac conduction system at most of these levels can give rise to significant, symptomatic, and often life-threatening bradyarrhythmias. Collaborative efforts of biomedical engineers and clinicians led to the advent of cardiac pacemakers. Initially designed as asynchronous pacing devices for Stokes-Adams attacks, these devices now are fine microprocessors that transmit electrical information back and forth to the heart, store vast amounts of diagnostic and therapeutic information, monitor and regulate their own performance and function, prevent and pace-terminate tachyarrhythmias, and raise alarms when doubts about their malfunction arise.
Pacemakers are an important therapy not only of bradyarrhythmias, but also of other clinical situations based on newly defined roles. Before implantation, the clinician should justify its indication and define the immediate and long-term pacing needs of the patient. With the availability of a wide variety of pacemakers, pacing modes, diagnostic and therapeutic tools, and programming options, a thorough and updated knowledge is important to achieve maximum clinical benefit for the patient.
Pacemakers can be single- or dual-chamber devices. Dual-chamber devices have capabilities for biventricular or biatrial pacing. The expanding role of biventricular devices is considered in greater detail in the chapter on cardiac resynchronization therapy. When determining the most appropriate pacing mode for a patient, the clinician should consider the patient’s activity level, need for AV synchrony, need for chronotropic support, need and possibility for preservation of intrinsic AV node conduction, presence of atrial or ventricular arrhythmias, and associated medical conditions.
Pacemaker Nomenclature
A three-letter code describing the basic function of the various pacing systems was first proposed in 1974 and updated in 2002. Designated the NBG code for pacing nomenclature (1) (Table 74.1), the code has five positions and is generic rather than pertaining to a specific device.
TABLE 74.1 Pacemaker Nomenclature | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||
The first position refers to the chamber(s) in which stimulation occurs: A, atrium; V, ventricle; O, none; and D, dual chamber, that is, both A and V. The second position refers to the chamber(s) in which sensing occurs. The letters are the same as those for the first position. Manufacturers may also use S in both the first and second positions to indicate that the device is capable of pacing or sensing only a single cardiac chamber. The third position refers to how the pacemaker responds to a sensed event. An I indicates that a sensed event inhibits the output pulse and causes the pacemaker to recycle for one or more timing cycles. A T means that an output pulse is triggered in response to a sensed event. A D means that both T and I responses can occur. This designation is restricted to dual-chamber systems. An event sensed in the atrium inhibits the atrial output but triggers a ventricular output. Unlike a single-chamber–triggered mode (VVT or AAT), in which an output pulse is triggered immediately on sensing, there is a delay between the sensed atrial event and the triggered ventricular output to mimic the normal PR interval. If a native ventricular signal or R wave is sensed, it inhibits the ventricular output and possibly even the atrial output, depending on where sensing occurs.
The fourth position of the code reflects both programmability and rate modulation. An R in the fourth position indicates that the pacemaker incorporates a sensor to control the rate independent of intrinsic cardiac activity. Practically, R is the only indicator used in the fourth position, but other indicators described are shown in Table 74.1. The fifth position of the code has been changed and is used to describe whether multisite pacing is present in none of the chambers (O), in the atria (A), or in the ventricles (V). Hence, for a patient with multisite ventricular pacing available with rate responsiveness, the code is DDDRV.
Glossary of Pacing Modes and Timing Cycles
AAI/AAIR
Single-chamber atrial pacing modes, with R denoting the capability of rate adaptation.
Atrioventricular interval (AVI)
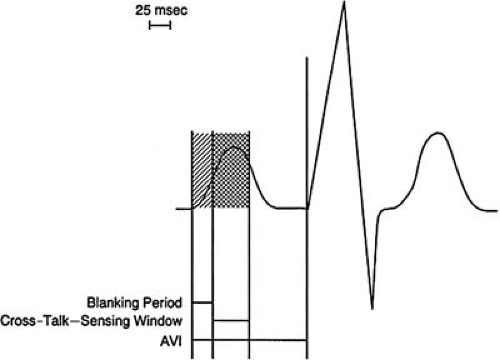
The atrioventricular interval in a dual-chamber pacemaker is the programmed duration following a sensed or paced atrial beat that is allowed before a ventricular pacing impulse is delivered. The initial portion of this interval is the atrial blanking period. This is immediately followed by the cross-talk sensing window, which is the timing during which cross-talk can occur due to afterpotentials from an atrial output (Fig. 74.1).
Blanking period
An interval during which the sensing circuit of the pacemaker is temporarily disabled following the delivery of an output pulse. This prevents inappropriate sensing of residual energy from the pacemaker output pulse and, in dual-chamber pacemakers, prevents sensing of pacemaker output pulses or intrinsic events in the chamber other than that in which the event occurs. Hence, there could be an atrial or a ventricular blanking period in response to an atrial or ventricular output, respectively. In a dual-chamber pacemaker, the postventricular atrial blanking period (PVAB) refers to the period during which the atrial circuit is disabled after a ventricular sensed or paced event.
Cross-talk
In dual-chamber pacemakers, after delivery of a pacing impulse in one chamber, the afterpotentials may be of sufficient strength and duration to be sensed in the other chamber. This is referred to as cross-talk. It can rarely be seen in the ventricles after an atrial impulse but is less likely to occur in the atrium because it may be buffered by the PVAB or the PVARP.
DDD/DDDR
Dual-chamber pacing modes, with R denoting the capability of rate adaptation.
Functional pacing abnormalities (e.g., functional undersensing or functional failure to capture)
Failure to sense or capture that is appropriate because of imposed pacemaker refractory periods or myocardial refractoriness due to a previous intrinsic atrial or ventricular event.
Refractory period
The interval during which a given sensing circuit detects but does not respond to any sensed event in that chamber, that is, the atrial or ventricular refractory periods. During the postventricular atrial refractory period (PVARP), the atrial-sensing circuit is refractory following a sensed or paced ventricular event. The purpose of this is to avoid sensing of retrograde atrial activity after ventricular activation that could initiate an endless-loop tachycardia.
Total atrial refractory period
The period during which the atrial channel of a dual-chamber or VDD pacemaker ignores intrinsic atrial activity or other activity sensed on the atrial-sensing circuit. This is usually a combination of the atrioventricular interval and the postventricular atrial refractory period.
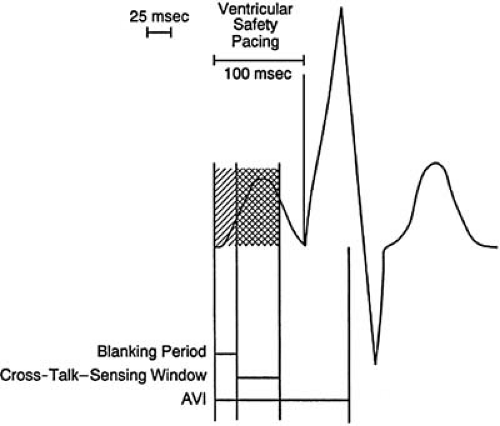
Ventricular safety pacing
The delivery of a ventricular output pulse, following atrial pacing, if a signal is sensed by the ventricular channel during the early portion of the AV interval (AVI), which is the cross-talk window. This is to ensure that ventricular depolarization is not inhibited by cross-talk and occurs even if the sensed event was something other than an intrinsic ventricular depolarization (Fig. 74.2).
VVI/VVIR
Single-chamber ventricular pacing modes, with R referring to the capability of rate adaptation.
Indications of Cardiac Pacing
The American Heart Association/American College of Cardiology (AHA/ACC) guidelines classify pacing indications into three categories, namely those in which pacing is generally indicated, may be indicated, and is not indicated (2). The most common are bradyarrhythmic causes such as those from sinus node dysfunction, neurocardiogenic syncope, acquired or congenital AV block, chronic bifascicular, and trifascicular block. The nonbradycardic causes of pacing such as atrial fibrillation, hypertrophic and dilated cardiomyopathy, congestive heart failure, and long-QT syndrome are discussed separately.
Sinus Node Dysfunction
Significant sinus bradycardia is generally accepted as rate less than 40 beats per minute during waking hours. Pacing is indicated in patients with symptomatic or significant sinus node dysfunction. Sinus bradycardia, sinus pauses or arrest, and sinoatrial exit block are the usual variants of sinus node dysfunction. The rate at which pacing is indicated is debatable, but in general it is considered beneficial in patients with symptoms consistent with bradycardia. Although every patient needs to be considered individually, most clinicians agree that sinus pauses of 3 seconds or more during waking hours should be considered abnormal and may warrant pacing. Pauses that occur during sleep are more difficult to categorize. Because of vagal influences, many healthy persons may have pauses longer than 3 seconds during sleep. In the absence of symptoms or rhythm disturbances during waking hours, this should not require treatment. Permanent pacing should be considered for any patient who has symptomatic bradyarrhythmia when the cause is not reversible. Reversible sinus node dysfunction can be noted in situations such as the postoperative stages of cardiac surgery, rarely after an acute myocardial infarction, or a myocarditis.
Permanent pacing for patients with sinus node dysfunction after myocardial infarction is reserved for those who have symptoms. If drug therapy results in symptomatic bradycardia, criteria for permanent pacing should follow the guidelines given for sinus node dysfunction in Table 74.2. Paroxysmal episodes of atrial arrhythmias, most commonly atrial fibrillation and atrial flutter, can be interspersed with periods of significant sinus node dysfunction, the commonly noted diagnosis of tachycardia-bradycardia syndrome. This is most notable in the postconversion phase after either a spontaneous or an electrical cardioversion. Apart from producing symptoms, it makes drug therapy a more challenging task. Such patients are often best managed with conjunctive pacemaker therapy. Patients with chronic atrial fibrillation could also have slow ventricular rates due to AV nodal–blocking drugs or complete AV block, in either situation requiring a pacemaker.
Another manifestation of sinus node dysfunction could be the inability to achieve at least 80% of the predicted heart rate with exercise, leading to chronotropic incompetence. In such situations the resting heart rates may be normal, but patients become symptomatic with effort and can improve significantly with rate-responsive pacing.
Various clinical trials studied the relative benefits of single- and dual-chamber pacemakers in patients with sinus node dysfunction. Some of these trials also included patients with AV block. In a small study of 225 patients, Andersen et al. (3) were the first to suggest that atrial pacing may be superior to ventricular pacing in patients with sinus node dysfunction. They showed a reduction in atrial fibrillation, thromboembolism,
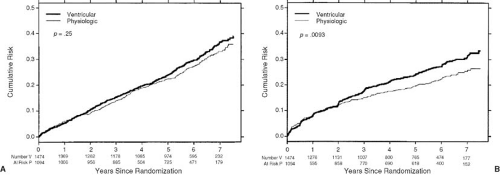
and cardiovascular mortality in patients with atrial pacing compared with ventricular pacing. However, larger trials, like the Canadian Trial of Physiologic Pacing (CTOPP) (4) and the MOde Selection Trial in sinus node dysfunction (MOST) (5), did show a lower incidence of atrial fibrillation with atrial or dual-chamber pacing but failed to show any significant benefit in terms or cardiovascular mortality or the risk of stroke. These effects were sustained even on longer follow-up (6) (Fig. 74.3). In patients older than the age of 65 years, the PASE (Pacemaker Selection in the Elderly) trial showed no change in the quality of life, mortality, atrial fibrillation (AF), or stroke (7). In the ongoing Danish Pacing Trial (DANPACE) (8), patients with tachycardia-bradycardia syndrome with normal AV conduction are being assessed for any differences in all-cause and cardiovascular mortality, AF, quality of life, incidence of atrial fibrillation and thromboembolism, and cost-effectiveness with AAIR versus DDDR pacing.
and cardiovascular mortality in patients with atrial pacing compared with ventricular pacing. However, larger trials, like the Canadian Trial of Physiologic Pacing (CTOPP) (4) and the MOde Selection Trial in sinus node dysfunction (MOST) (5), did show a lower incidence of atrial fibrillation with atrial or dual-chamber pacing but failed to show any significant benefit in terms or cardiovascular mortality or the risk of stroke. These effects were sustained even on longer follow-up (6) (Fig. 74.3). In patients older than the age of 65 years, the PASE (Pacemaker Selection in the Elderly) trial showed no change in the quality of life, mortality, atrial fibrillation (AF), or stroke (7). In the ongoing Danish Pacing Trial (DANPACE) (8), patients with tachycardia-bradycardia syndrome with normal AV conduction are being assessed for any differences in all-cause and cardiovascular mortality, AF, quality of life, incidence of atrial fibrillation and thromboembolism, and cost-effectiveness with AAIR versus DDDR pacing.
TABLE 74.2 Recommendations for Permanent Pacing in Patients with Sinus Node Dysfunction | ||
|---|---|---|
|
With the recent recognition of the importance of dyssynchrony in patients with heart failure and the benefits of resynchronization therapy (9,10), electrophysiologists have become increasingly aggressive in avoiding inadvertent ventricular pacing in patients with intact AV node function. This is especially true for patients with existing left ventricular dysfunction, in whom the reasons for avoiding pacing-induced dyssynchrony are more obvious. The increased mortality associated with the DDDR mode in implantable cardioverter-defibrillator (ICD) patients, most likely from ventricular pacing, has been established in the DAVID trial (11). When patients with left ventricular dysfunction are given dual-chamber devices for sinus node dysfunction, aggressive efforts are made to prolong the AV
delay enough to promote intrinsic conduction in patients with normal AV node function. Even in patients with borderline AV node function, newer devices use algorithms like AV search hysteresis, auto-intrinsic search, and managed ventricular pacing (MVP) to avoid ventricular pacing as much as possible.
delay enough to promote intrinsic conduction in patients with normal AV node function. Even in patients with borderline AV node function, newer devices use algorithms like AV search hysteresis, auto-intrinsic search, and managed ventricular pacing (MVP) to avoid ventricular pacing as much as possible.
TABLE 74.3 Indications for Cardiac Pacing in Patients with Carotid Sinus Hypersensitivity and Neurocardiogenic Syncope | ||
|---|---|---|
|
Carotid Sinus Hypersensitivity and Neurocardiogenic Syncope
Although they are not related to any intrinsic sinus node dysfunction, these two syndromes could result in profound inhibition of the SA node. Both are characterized by a variable degree of cardioinhibitory and vasodepressor response, and most often a combination of the two. Permanent pacemakers may be indicated in patients with such neurally mediated syncope (12) and are especially useful in patients with a cardioinhibitory response.
In patients with carotid sinus hypersensitivity (CSH), carotid stimulation could produce sinus arrest, sinus pauses of 5 to 10 seconds, or even AV block lasting for greater than 3 seconds. In patients with a vasodepressor response, there could be a symptomatic fall in the systolic blood pressure by more than 50 mm Hg. Many patients may have a mixed response. For patients with a cardioinhibitory response, a permanent pacemaker could be beneficial (13) if they do not have improvement after removal of precipitating factors such as shaving, wearing tight collars, looking up, being subjected to the Valsalva maneuver, and so on. Because the intense vagal output may often cause AV block, the backup of ventricular pacing is essential, and hence these patients need a dual-chamber pacemaker.
The recommendations for pacing in patients with neurocardiogenic syncope have undergone significant evolution. As discussed in other chapters, patients with neurocardiogenic syncope can also have a cardioinhibitory, vasodepressor, or mixed response. In the initial North American Vasovagal Pacemaker Study I (VPS-I) (14), the trial had to be prematurely terminated due to the significantly lower incidence of syncope in patients with pacemakers versus those without pacing (17% vs. 59%). The Vasovagal Syncope International Study (VASIS) (15) also showed reduced syncopal episodes in patients with pacemakers (5% vs. 61%). Even when compared with β-blockers, which are often considered the first-line therapy, pacemakers were shown to be superior in the Syncope Diagnosis and Treatment Study (16). In contrast, the Vasovagal Syncope and Pacing study (SYNPACE) (17) and the more recent Vasovagal Pacemaker Study II (VPS II) (18) failed to show any superiority of the pacemaker therapy. Unlike the other trials, in these two trials, pacemakers were implanted even in the control group of patients but were essentially programmed to a nonpacing mode. This suggests that part of the benefit in the other trials may have been a placebo effect. The ongoing Syncope and Falls in the Elderly Pacing and Carotid Sinus Evaluation (SAFE-PACE 2) study is also looking for the possible benefits of permanent pacing in patients with neurocardiogenic syncope (19).
The recommendations for pacing in these two syndromes are summarized in Table 74.3. Because these patients have a sudden drop in heart rate, algorithms such as “rate drop,” “rate hysteresis,” “rate smoothing,” “flywheel,” and so on are useful in enhancing the benefits of a pacemaker in these situations. These options prevent a sudden drop in heart rate to the lower rate of the pacemaker and can prevent symptoms not only of absolute, but also of relative bradycardia.
Chronic Bifascicular or Trifascicular Block
Disorders of conduction at the level of the branches of the His-Purkinje system are referred to as bundle branch blocks or intraventricular conduction defects. In patients with isolated right- or left-bundle-branch block (RBBB or LBBB, respectively), the risk of progression to advanced AV block is rare and pacing is not usually indicated. Patients with a bifascicular block (RBBB with a left anterior or posterior hemiblock or a LBBB with a left-axis deviation) have a 6% incidence of progression to complete heart block (20). If such patients have intermittent advanced AV block, alternating RBBB and LBBB, or complete heart block, pacing is indicated even if they are asymptomatic. If these patients have symptoms like presyncope/syncope without a demonstrable cause, it continues to be a class II indication for pacing. Permanent pacing may also be beneficial for patients with evidence of His-Purkinje disease on electrophysiologic study [e.g., an HV interval ≥100 msec, or infra His block with atrial pacing (21)]. The recommendations for pacing in patients with fascicular block as per the 2002 guidelines are summarized in Table 74.4.
The development of a new intraventricular conduction disturbance with a first-degree AV block in a patient with acute myocardial infarction (MI) has a 40% risk of progression to complete heart block. In such patients, temporary pacing is certainly indicated, and many believe that permanent pacing is also needed (22). Intraventricular conduction disturbances
carry a poor prognosis in the presence of neuromuscular dystrophies or with structural heart disease such as ischemic or dilated cardiomyopathy. In a large majority of these patients, ICD or cardiac resynchronization therapy (CRT) may be indicated (as discussed in other chapters). In others, a close watch may be warranted due to the unpredictable rate or progression of the disease. For fascicular blocks in asymptomatic patients with normal hearts, pacing is not indicated. The recommendations for permanent pacing in patients with an acute MI are summarized in Table 74.5.
carry a poor prognosis in the presence of neuromuscular dystrophies or with structural heart disease such as ischemic or dilated cardiomyopathy. In a large majority of these patients, ICD or cardiac resynchronization therapy (CRT) may be indicated (as discussed in other chapters). In others, a close watch may be warranted due to the unpredictable rate or progression of the disease. For fascicular blocks in asymptomatic patients with normal hearts, pacing is not indicated. The recommendations for permanent pacing in patients with an acute MI are summarized in Table 74.5.
TABLE 74.4 Recommendations for Permanent Pacing in Patients with Chronic Bifasicular and Trifascicular Block | ||
|---|---|---|
|
Congenital Complete Heart Block
There are various reasons for the gradual decline in the threshold used to decide whether to pace patients with congenital complete heart block even if they are asymptomatic. They have been shown to have unpredictable syncope, significant mortality, gradual decline in heart rates, and a high incidence of acquired mitral regurgitation. The timing for pacemaker insertion has always been a question.
TABLE 74.5 Recommendations for Permanent Pacing after the Acute Phase of Myocardial Infarction | ||
|---|---|---|
|
In pediatric patients with congenital complete heart block, pacemaker implantation is recommended for patients with congestive heart failure, patients with an average heart rate less than 50 beats per minute while awake, and patients with a history of syncope or presyncope, significant ventricular ectopy, or exercise intolerance (23). In adults, we recommend pacing in all patients with congenital complete heart block due to the foregoing factors. The other indications for pacing in patients with congenital heart disease as per the ACC guidelines are summarized in Table 74.6.
Acquired Atrioventricular Block
Traditionally, AV block is classified as first-, second,- or third-degree (also complete) heart block. Alternatively, it can be defined anatomically/physiologically as supra-, intra-, or
infra-Hisian. If the QRS is wide and the conducted P waves have a normal PR interval, there is a greater probability that the conduction disturbance is infra-Hisian. Most commonly, acquired AV block is idiopathic and related to aging, but it has many potential causes, as discussed in other chapters. The recommendations for permanent pacing in acquired AV block are listed in Table 74.7. In general, pacing is indicated in all patients with complete heart block, symptomatic patients with second-degree AV block, and asymptomatic patients with advanced second-degree AV block if the pauses are greater than 3 seconds or the escape rates are less than 40 beats per minute when awake.
infra-Hisian. If the QRS is wide and the conducted P waves have a normal PR interval, there is a greater probability that the conduction disturbance is infra-Hisian. Most commonly, acquired AV block is idiopathic and related to aging, but it has many potential causes, as discussed in other chapters. The recommendations for permanent pacing in acquired AV block are listed in Table 74.7. In general, pacing is indicated in all patients with complete heart block, symptomatic patients with second-degree AV block, and asymptomatic patients with advanced second-degree AV block if the pauses are greater than 3 seconds or the escape rates are less than 40 beats per minute when awake.
TABLE 74.6 Recommendations for Permanent Pacing in Patients with Congenital Heart Disease | ||
|---|---|---|
|
Indications for permanent pacing for AV block that occurs with acute myocardial infarction are more controversial. Generally, a pacemaker is indicated for complete AV block, Mobitz II AV block, or bilateral and alternating bundle branch blocks that persist longer than 72 hours after the acute event. Some clinicians consider new and persistent bifascicular block an indication for pacing, whether or not it is associated with transient supra- or infra-Hisian AV block. Such criteria may be more lenient after an anterior wall MI because conduction disturbances after an inferior wall MI have a better prognosis. An isolated hemiblock of one of the left-sided fascicles, an isolated transient AV conduction disturbance without a residual intraventricular conduction disturbance, transient AV conduction disturbance with new-onset left anterior or left posterior hemiblock, or a first-degree or Mobitz I block with a preexisting bundle branch block are more gray areas, but would generally not be strong indications for a pacemaker in an asymptomatic patient.
It would appear that dual-chamber pacing is the most physiologic mode of pacing in patients with AV block. With the known adverse effects of right ventricular pacing, it may be wise to consider options to avoid ventricular pacing as much as possible in patients with left ventricular dysfunction if they have intermittent AV block. In patients with class III and IV heart failure, it may be reasonable to consider a biventricular pacemaker if they have a left ventricular ejection fraction (LVEF) of 35% or less and a left ventricular end-diastolic dimension (LVEDD) of greater than 55 mm.
Even in patients with a normal LV systolic function, dual-chamber pacing appears to be most physiologic. The CTOPP (6) and PASE (7) trials discussed earlier included patients with sick sinus syndrome and AV block. Although these trials failed to show any benefit in survival, there were significant improvements with regard to quality of life and incidences of atrial fibrillation and thromboembolism. The United Kingdom Pacing and Cardiovascular Events (UKPACE) trial (24) included patients with AV block older than the age of 70 years and studied the effect of dual-chamber versus single-chamber pacemakers
on all-cause mortality and the composite endpoints of cardiovascular deaths, heart failure hospitalizations, atrial fibrillation, strokes, and reoperations. No difference was observed in any of the endpoints in the two groups.
on all-cause mortality and the composite endpoints of cardiovascular deaths, heart failure hospitalizations, atrial fibrillation, strokes, and reoperations. No difference was observed in any of the endpoints in the two groups.
TABLE 74.7 Recommendations for Permanent Pacing in Patients with Acquired Block in Adults | ||
|---|---|---|
|
One could argue that most of the patients enrolled in these trials were of a much older age. Our policy is to offer a dual-chamber pacemaker to all patients with complete heart block unless they have underlying chronic atrial fibrillation with no plans to pursue a rhythm control strategy. This is especially true for younger patients. The atrial lead could be beneficial in providing an additional atrial boost, avoiding pacemaker syndrome, and also taking care of any accompanying sinus node dysfunction. In addition to physiologic pacing, it also monitors the rhythm disturbances in the atrium.
Nonbradyarrhythmic Indications for Pacing
Hypertrophic Obstructive Cardiomyopathy
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree