Cardiac Catheterization and Percutaneous Intervention
Angela M. Palazzo
Neel P. Chokshi
David L. Coven
CORONARY ANGIOGRAPHY
CORONARY ANATOMY
Ample arterial circulation is required for effective myocardial function during both systole and diastole. This supply/demand coupling is accomplished through regional matching of the arterial supply to a particular portion of the myocardium.1
Arterial circulation of the heart consists of two parts: (1) large epicardial coronary arteries that serve as conduit vessels, and (2) medium-sized and small intramyocardial coronary arterioles that serve as resistance vessels regulating the amount of coronary flow according to myocardial metabolic needs. Perturbation in any portion of this arterial tree will lead to regional myocardial dysfunction. Acute coronary syndrome (ACS) is the clinical manifestation of a diminished coronary arterial blood supply in conduit or resistance coronary vessels with atherosclerosis being the most common cause.
In most humans, the entire epicardial circulation originates from the two initial branches of the aorta: the left coronary artery (LCA) and the right coronary artery (RCA). They originate from the left and the right sinus of Valsalva, respectively. The initial portion of the LCA is referred to as the left main coronary artery (LMCA); it branches into the left anterior descending artery (LAD) and the left circumflex artery (LCx).
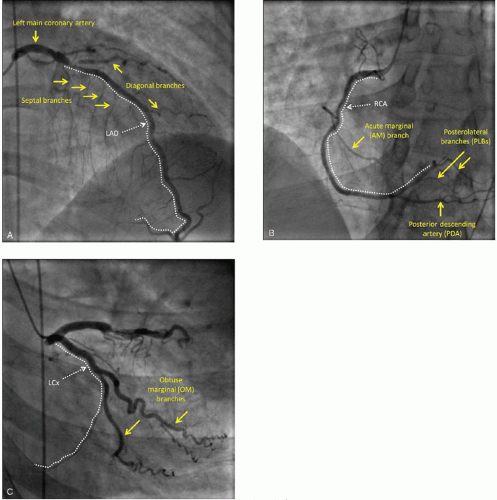
In a few individuals, there may be anomalies in the origin of the coronary arteries pertaining to the number and location of coronary ostia within the aortic root as well as anomalies in the initial course of the vessels. The LAD supplies the largest portion of the left ventricle; the size of the LAD territory tends to be relatively constant among individuals and encompasses about 50% of the left ventricle. The LAD initially runs in the anterior interventricular groove parallel to the long axis of the heart then turns over the left ventricular apex and terminates, in most individuals, in the apical region of the posterior interventricular groove. The LAD gives off septal branches that penetrate into the anterior two-thirds of the interventricular septum, and diagonal branches which supply large areas of the anterior wall of the left ventricle (Figure 4.1A).
The other half of the left ventricle is supplied by both the RCA and the LCx in proportions that vary between individuals. In about 70% of humans, the RCA subtends a larger section of the left ventricle than the LCx (the so-called right-dominant circulation). In about 20% of individuals, the contribution of the two arteries is equal (co-dominant or balanced circulation). In the remaining 10%, the LCx is larger than the RCA (left-dominant circulation). The dominance type does not affect the initial course of either the RCA or the LCx; it arises from the pattern of terminal branching in the two vessels.
In all individuals, the initial course of the RCA is within the right atrioventricular groove and thus perpendicular to the long axis of the heart. During this initial course, the RCA gives off acute marginal (AM) branches that run roughly parallel to the long axis of the right ventricle to supply the acute margin of the right ventricle. The RCA is the principal source of arterial blood supply to the right ventricle; when there is right ventricular dysfunction during ACS, it is invariably caused by abnormalities in the RCA tree (Figure 4.1B).
In a roughly mirror-image pattern, the LCx initially runs in the left atrioventricular groove perpendicular to the long axis of the heart and gives off obtuse marginal (OM) branches. They run parallel to the long axis of the heart and supply the obtuse margin of the heart made up by the lateral wall of the left ventricle (Figure 4.1C).
The inferoposterior aspects of the interventricular septum and the left ventricle are supplied by the posterior descending artery (PDA) and one or more posterolateral branches (PLBs). The PDA usually runs along the proximal two-thirds of the posterior interventricular groove, and its course is parallel to the LAD in the anterior interventricular groove. Along its interventricular course, the PDA gives off septal branches to the inferior aspect of the interventricular septum and meets the LAD in the apical portion of the posterior interventricular septum. PLBs are arterial branches that run along the long axis of the left ventricle and roughly parallel to the course of the PDA and the OMs. PLBs supply the inferior and posterior walls of the left ventricle.
It is the origin of the PDA that determines whether the coronary circulation is right dominant, left dominant, or balanced (co-dominant). In the right-dominant circulation, the PDA is a branch of the RCA; in the left-dominant circulation the PDA is a terminal branch of the LCx; in balanced (co-dominant) circulation both the RCA and the LCx supply the PDA.
PREPROCEDURAL MATTERS
Patient consent.
Prior to the procedure, the physician performing the angiography should obtain informed consent from the patient. The discussion should include the indication, the general nature of the procedure, and the associated risks. The specific risks include but are not limited to the following:
Bleeding
Infection
Allergic reaction to the contrast
Contrast induced nephropathy
Stroke
Myocardial infarction
Death
Medications.
The patient’s active medication list should be reviewed prior to angiography. Individuals on metformin should have this medication held for at least 48 hours post-procedure for the theoretical risk of contrast nephropathy and subsequent lactic acidosis because of the medication. If preexisting renal insufficiency is present, metformin should not be given 48 hours before the procedure as well.
Allergies.
Patients should be asked specifically regarding allergies to contrast. If a patient endorses a reaction to this agent, the risks of contrast administration should be weighed prior to proceeding. In instances of a mild reaction, patients can generally proceed after premedication with diphenhydramine without further problems.
Laboratory testing.
When possible, all patients should have a recent laboratory evaluation including assessment of renal function, baseline hemoglobin level, and coagulation studies. Although this may not be feasible in the emergent setting, labs should be at least drawn prior to the procedure and monitored afterwards. In patients with renal disease, the risks of contrast
nephropathy should be considered prior to proceeding and intravenous fluids should be given N-acetylcysteine should be considered if not contraindicated.
nephropathy should be considered prior to proceeding and intravenous fluids should be given N-acetylcysteine should be considered if not contraindicated.
Electrocardiogram.
The electrocardiogram (ECG) should be reviewed for abnormalities that may be suggestive of an anatomical site of myocardial injury. This may be useful in establishing the culprit coronary vessel and guiding intervention.
Anesthesia.
Local anesthesia is generally used at the site of arterial puncture to minimize pain. Conscious sedation can also be administered to reduce anxiety and relieve pain. A variety of short-acting agents can be used.
Arterial access.
To perform coronary angiography, the coronary ostia must be directly cannulated with a catheter at the aortic root. This catheter is inserted into the arterial vasculature peripherally and guided up to the root of the aorta under fluoroscopy. To obtain access, an arterial sheath (Figure 4.2) is placed using a modified Seldinger technique at one of three possible peripheral sites. The femoral artery is the most common site of peripheral access for coronary angiography. The sheath is placed in the common femoral artery proximal to the bifurcation into the superficial femoral and profunda femoris arteries and distal to the superficial epigastric artery. Relative to anatomical landmarks, the puncture is made over the palpable pulse just inferior to the inguinal ligament at the level of the midpoint of the femoral head. The large lumen size of this vessel segment minimizes the risk of acute occlusion during the procedure, and the vessel’s superficial course allows direct compression for closure of the puncture site. As an alternative to the femoral artery in the leg, cardiac catheterization can be performed either from the brachial artery or from the radial artery in the arm. Both approaches allow for almost immediate ambulation of the patient.
Brachial artery cannulization can accommodate larger diameter catheters but the risk of bleeding is higher than with a radial approach and occlusion of the brachial artery compromises the dual circulation to the hand.
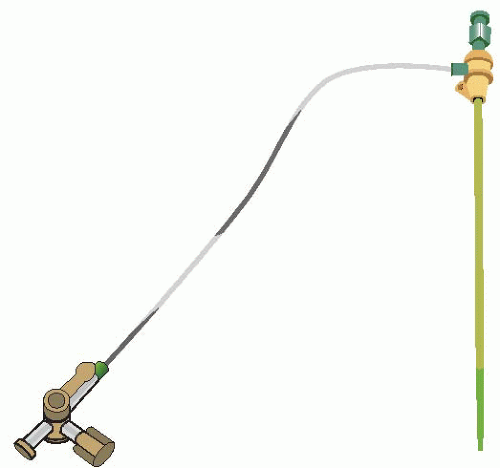 Figure 4.2. An arterial sheath, shown here, is placed in the peripheral artery. This serves as the point of access to perform the cardiac catheterization. |
Radial artery access has the lowest bleeding risk of any site. Prior to the performance of a radial catheterization, an Allen’s test must be performed to insure that dual circulation to the hand is intact in the event of a radial occlusion. A micropuncture needle is used for cannulation. Vasospasm of the radial artery sometimes occurs and pretreatment through the sheath with nitroglycerin and verapamil can often mitigate this.
For either approach, the sheath is removed immediately following the study and manual compression is applied.
Diagnostic catheterization.
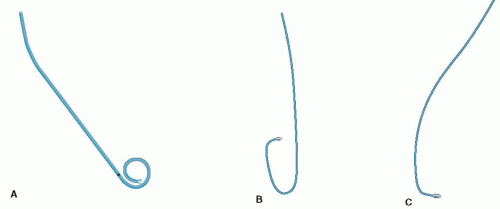
With the arterial sheath in place, a guide wire and catheter are inserted into the sheath and advanced from the peripheral artery to the level of the aortic root under fluoroscopy. The wire is then withdrawn, and the catheter is manipulated by the angiographer to visualize the desired coronary artery or the left ventricle. Typically, left ventriculography is performed using a pigtail catheter (Figure 4.3A). Cannulation and visualization of the right and left coronaries are done with their respective catheters (Figure 4.3B, C).
LEFT VENTRICULOGRAPHY.
Once at the level of the aortic root, the pigtail catheter is advanced across aortic valve and positioned in the center of the left ventricle. Contrast is injected into the ventricle while recording the radiographic images in a right anterior oblique (RAO) view. This provides a two-dimensional image of the anterior, inferior, and apical walls of the left ventricle (Figure 4.4A, B). Images may also be obtained in the left anterior oblique (LAO) view for visualization of the posterior wall and the interventricular septum. This image modality is useful in assessing wall motion abnormalities and ejection fraction as well as mitral regurgitation as contrast can be directly visualized leaking across the mitral valve. In patients with kidney disease, the left ventriculogram may not be performed to minimize exposure to nephrotoxic contrast.
LEFT HEART PRESSURES.
With the pigtail catheter in the left ventricle, intracavitary pressures tracings can be directly measured including the left ventricular end diastolic pressure (LVEDP) which is often useful in the clinical assessment of patients with ACS. Patients can also be evaluated for aortic stenosis by assessing the transvalvular pressure gradient as the catheter is pulled back across the aortic valve.
CORONARY ARTERIES.
Assessment of the coronary arteries is performed by cannulating the artery, injecting contrast under fluoroscopy, and assessing the opacification of the artery to identify narrowing and irregularities in contrast flow. The degree of stenosis is quantified based on the lumen width ahead of and following the site of narrowing. The degree of stenosis can also be assessed by grading the quality of contrast flow distal to the site of narrowing.
CORONARY ARTERY BYPASS GRAFTS.
In patients with previous coronary bypass surgery, grafts are cannulated and visualized with a variety of specialty catheters. It is helpful to know the patient’s bypass graft anatomy prior to the procedure as this can guide the angiographer in searching for saphenous grafts and internal mammary grafts.
Closure of puncture site.
Following cardiac catheterization, all sheaths and catheters are removed and hemostasis is achieved
in a variety of ways. Direct manual pressure can be applied to the arterial site for a minimum of 10 minutes to control the bleeding. Sheath size and anticoagulation status may greatly increase the time necessary to obtain adequate hemostasis. Various compression devices are marketed and are usually used as an adjunct to manual compression when anticoagulation requires longer compression period. Although of different designs, they all exert external pressure over the arteriotomy site. It is important to note that all external compression devices require a high level of vigilance to assure that the device is properly placed and bleeding is successfully controlled.
in a variety of ways. Direct manual pressure can be applied to the arterial site for a minimum of 10 minutes to control the bleeding. Sheath size and anticoagulation status may greatly increase the time necessary to obtain adequate hemostasis. Various compression devices are marketed and are usually used as an adjunct to manual compression when anticoagulation requires longer compression period. Although of different designs, they all exert external pressure over the arteriotomy site. It is important to note that all external compression devices require a high level of vigilance to assure that the device is properly placed and bleeding is successfully controlled.
A number of devices to close the arteriotomy site directly are currently on the market. These include, but are not limited to, the Angio-seal vascular closure device (St. Jude Medical) that has a bioabsorbable intravascular layer and a collagen extra-vascular plug that act together to seal the puncture site. After 60 to 90 days, these components dissolve, and the access site can be used again. A possible complication unique to this device is embolization of the intravascular component. The Mynx vascular closure device (AccessClosure) is a sealant that is introduced into the tissue tract above the arteriotomy site. When exposed to blood this sealant swells to achieve hemostasis. The third example of arteriotomy closure devices is the Perclose device (Abbott Laboratories) that deploys needles percutaneously to suture the arteriotomy site. A possible complication of this device is inadvertent complete ligation of the artery.
Although the time to ambulation is improved using these closure devices, there is scant evidence to suggest that complications rates are reduced. Patient comfort has been cited as an advantage to their usage.