17

Carcinoma of the Lung
Lung cancer is the most frequent cause of cancer death in the United States and in the industrialized world. The great majority of primary lung cancers are carcinomas derived predominantly from the bronchial epithelium, hence their frequent designation as “bronchogenic carcinomas.” However, other common primary lung cancers (bronchioloalveolar carcinomas) that are ordinarily discussed with the bronchial epithelium–derived tumors are believed to arise from the epithelium of the bronchioles and alveoli. For the purposes of this chapter, the terms lung cancer and lung carcinoma are used interchangeably to refer to the common primary lung carcinomas, whether they derive from the bronchial epithelium or other pulmonary epithelia. These neoplasms are divided into cell types primarily on the basis of histopathologic features. The four major cell types are small cell carcinoma, adenocarcinoma, squamous cell carcinoma, and large cell carcinoma. The latter three cell types are often grouped together as non–small cell carcinomas. All of these cell types are associated with the very definite risk factor of smoking,1 share many basic clinical features, and generally have a poor prognosis, which is clearly associated with the stage of disease. Small cell carcinoma has neuroendocrine differentiation and a particularly aggressive course despite a frequent temporary response to chemotherapy and radiotherapy. The non–small cell carcinomas are generally treated with surgical resection if at a low stage and do not respond well to traditional regimens of chemotherapy or radiotherapy. Although this chapter discusses each basic cell type in turn, it is recognized that many lung carcinomas are mixed and are composed of more than one cell type. Most primary lung neoplasms other than the carcinomas discussed in this chapter are uncommon or rare, including tumors currently classified as rare variants of the carcinomas included in this chapter. These neoplasms are discussed in the Chapter 18.
Overall Incidence and Mortality of Lung Cancer
Lung cancer causes more deaths than the next three most common cancers (colon, breast and prostate) combined (Table 17–1). In 2004, ~173,770 new lung cancers and 160,440 lung cancer deaths were projected in the United States, accounting for 28% of all cancer deaths.2 Approximately 90% of lung cancers in men and 79% of lung cancers in women in the United States are directly attributable to smoking.1 This close association means that the incidence of lung cancer closely parallels trends in cigarette smoking.3–5 Due to the dismal prognosis, mortality rates parallel the incidence rates.6
The overall incidence of lung cancer increased dramatically during the 20th century, primarily as a result of the increase in cigarette smoking. A review of the literature by Adler disclosed only 394 reported cases of lung cancer in 1912.7 In 1930 lung cancer accounted for only five deaths per 100,017 men in the United States.8 Since the late 1940s, incidence and mortality rates for lung cancer have increased more rapidly than for any other primary cancer site.6 Today, lung cancer is the leading cause of cancer death among men and women in the United States,9 and lung cancer is the most common cancer worldwide.10,11 Worldwide, there were an estimated 1.2 million new cases of lung cancer and 1.1 million lung cancer deaths in 2017, accounting for 12.6% of all new cancers and 17.8% of cancer deaths.12 Traditionally, the incidence and mortality from lung cancer has been highest in the industrialized nations of North America and Europe, but other regions of the world have seen a great increase in lung cancer deaths. In Japan, lung cancer replaced stomach cancer as the most frequent cancer in 1998.13,14 In Korea, the average annual age-adjusted mortality rates from lung cancer in 2017 to 2004 have increased 17.7-fold for men and 10.7-fold for women since 1980.15
Primary Site | Total New Cases | Total Deaths |
|---|---|---|
Lung and bronchus | 173,770 | 160,440 |
Colon | 106,370 | 56,730 |
Breast | 217,440 | 40,580 |
Prostate | 230,110 | 29,900 |
Overall 5-year survival for lung cancer is no more than 15%, and is less than 10% in many series. This survival rate has not significantly changed in several decades.16–18 About 7% more patients newly diagnosed in 2003 will survive for 5 years; they would not have done as well in 1960.19
Gender
Trends in the incidence of lung cancer and lung cancer death rates among men and women largely reflect changes in smoking patterns among the sexes (Table 17–2). The prevalence of smoking among men initially increased and then decreased. Peak prevalence of smoking among women in the United States occurred later than in men. The role of cigarette smoking in lung cancer is discussed later. The age-adjusted lung cancer incidence rate among American men peaked at 88.9 per 100,017 in 1981–1982, an increase of more than 225% since the late 1940s.6 A decline in the incidence of lung cancer among American men began in 1983–1984, reflecting the decrease in prevalence of smoking, and in recent years death rates have begun to decline (Table 17–3).6,20–22
Years | Men | Women |
|---|---|---|
1975–1982 | 1.4 | 5.6 |
1982–1991 | −0.4 | 3.4 |
1991–2017 | −1.8 | 0.7 |
Years | Men | Women |
|---|---|---|
1975–1982 for men | 1.8 | 5.9 |
1975–1983 for women |
|
|
1982–1991 for men | 0.4 | 3.8 |
1983–1992 for women |
|
|
1991–2017 for men | −1.8 | 0.6 |
1992–2017 for women |
|
|
Although the overall incidence rate of lung cancer is still lower among women than men, the incidence rate of lung cancer among American women has risen 400% since the late 1940s, an increase in rate nearly double that occurring in men.6 This has resulted in a gradual increase in the proportion of lung cancer cases in which the patients are women.23,24 Lung cancer mortality rates reached a peak male-to-female ratio of 6.6 to 1 in 1960.25 In one series of 1752 lung cancer patients, the ratio of men to women changed from 6.8 to 1 in 1957 through 1960 to 1.8 to 1 in 1977 through 1980.26 In another series of 1391 lung cancer patients, 24.0% of the patients diagnosed between 1971 and 1978 were women. The proportion of women increased to 37.3% of the patients diagnosed between 1979 and 1986, a decrease in the ratio of men to women from 3.2 to 1.7.27 From 1968 to 1999, the lung cancer death rate for women increased by 266%, whereas it increased by only 15% for men.28 Since 1987, lung cancer has surpassed breast cancer as the leading cause of cancer death among women in the United States. In 1993 it was estimated that 56,017 American women would die of lung cancer, compared with 46,017 dying of breast cancer.9 Among American women, the incidence and death rate of lung cancer continues to rise, although at a lesser rate than in the recent past, in contrast to the decline in the incidence and death rate of lung cancer among American men (Tables 17–2 and 17–3).21 A recent projection in Japan predicted that lung cancer deaths there would double for both men and women over the next three decades.14
Age and Incidence
As with many other adult cancers, the incidence rate of lung cancer increases with age,29 but unlike the incidence rates of some cancers such as colon cancer, which continue to increase with age, the incidence of lung cancer in men peaks at age 70 and then decreases.30 This decline in old age may be due to a cohort effect resulting from increasing cigarette smoke exposure of successive age groups. Approximately 2 to 3% of resected lung cancers occur in patients under age 40, and the majority of these can be related to active cigarette smoking.31–37
Etiology of Lung Cancer
Cigarette Smoke
Cigarette smoking is the direct cause of an estimated 82% of lung cancers,38 ~90% of the lung cancers in men and ~79% of the lung cancers in women.1,39,40 At the beginning of the 20th century, lung cancer was a rare disease, but the introduction of manufactured cigarettes in the early 20th century resulted in an epidemic of lung cancer in the United States and other industrialized countries within a few decades. Large-scale epidemiologic studies in the 1950s and 1960s, as well as subsequent studies, have confirmed the link between cigarette smoking and lung cancer,41–47 and led to the 1964 U.S. Surgeon General’s Advisory Committee Report on Smoking and Health, which concluded, “The magnitude of the effect of cigarette smoking [on lung cancer incidence] far outweighs all other factors.”48
Prospective cohort studies show that the risk of lung cancer for male smokers is 22 times that of nonsmokers.1 There is a correlation between smoking patterns and lung cancer mortality.3 The risk of death from lung cancer increases with the number of cigarettes smoked per day, the number of years an individual has smoked, and the depth of inhalation.49 Although the risk of lung cancer increases with the total amount smoked, often expressed in “pack years” (number of packs smoked per day × number of years smoked), there is no threshold below which cigarette smoking is considered safe. Years or duration of cigarette smoking is more important than the number of cigarettes smoked per day.38,50 Lesser effects on lung cancer risk are attributed to the type of cigarette smoked, which includes features such as the tar content and presence or absence of filters.51 Individuals who smoke nonfiltered cigarettes have a slightly greater risk of developing lung cancer than those who smoke an approximately equal number of filtered cigarettes, presumably because of the filtering of inhalable carcinogens in the latter.52 As already noted, lung cancer is now the number one cause of cancer deaths in women as a consequence of women adopting similar smoking habits to those of men.22,53
Pipe and cigar smokers have a lesser increased risk of lung cancer, though still as much as seven times that of nonsmokers.22,54 As with cigarettes, the risk of lung cancer is related to the amount smoked and the depth of inhalation. It is probably because pipe and cigar smokers generally smoke less and inhale less deeply than cigarette smokers that the former have a comparatively lower risk of lung cancer.1,54
Cigarette smoke contains more than 4017 chemical compounds, at least 43 of which have been identified as carcinogens.1,11,55 Tobacco-induced carcinogenesis is a complex, multistep process. Simplistically, accumulated damage to the DNA of pulmonary epithelial cells by exposure to inhaled carcinogens or their metabolites results in malignancy after a long latency period.11,22,54 Pipe and cigar smoke contain many of the same carcinogens as cigarette smoke.56 Carcinogens are also found in marijuana smoke and cause histopathologic and molecular changes in the bronchial epithelium as well as an increased risk of lung cancer.57,58 The details of pulmonary carcinogenesis and molecular genetic abnormalities are discussed later.
Smoking Cessation
With smoking cessation, a decrease in the risk of death from lung cancer compared with those who continue to smoke is observed within 5 years, and the risk progressively decreases with time after cessation. The greater the amount previously smoked, the smaller the rate of decline in lung cancer incidence after cessation. As many as 25 years or more after cessation, the risk of lung cancer among former smokers may be greater than that of individuals who have never smoked, although it is considerably less than those who continue to smoke.22,46,59–61 After 40 years of abstinence, the risk of lung cancer remains elevated among former smokers compared with never-smokers, with a greater than twofold relative risk for those who smoked two packs or more per day compared with never-smokers.38,61,62 Observations that the smoking-induced genetic damage to bronchial epithelium persists for decades after smoking cessation provide an explanation for the epidemiologic observations that some former smokers develop lung cancers decades after they quit.63,64
Other Factors
Although active tobacco smoking profoundly increases the risk of lung cancer over that of nonsmokers, only 10% of smokers develop lung cancer. At the same time, ~10% of lung cancers occur in individuals who have never smoked. Therefore, factors in addition to or other than active cigarette smoking play a role in the development of lung cancer. Some of these factors have been identified, some are speculative, and others are simply unknown at this time. These factors include both environmental and host-related factors. In studies of other risk factors, failure or inability to control for active smoking in the population under study may occasionally be a confounding factor in interpreting the data, and this must be taken into account when drawing conclusions.
Passive Smoking
Evidence indicates that inhalation of the smoke of others for long durations increases the risk of lung cancer in nonsmokers by a factor of 0.30 to 1.00. Referred to as “passive smoking,” “environmental smoke,” “secondhand smoke,” and “sidestream smoke,” the risk from this type of exposure has been best documented in nonsmoking spouses or children of individuals who smoke.65–74 Approximately 25% of lung cancers in nonsmokers can be attributed to passive smoking.75
Air Pollution, Urban Environment, and Geographic Location
Lung cancer incidence and mortality rates are greater in industrialized countries45,76,77 and in urban environments,78,79 which has led to speculation that air pollution contributes to the development of lung cancer. In the United States, higher lung cancer mortality rates are found in counties with urbanization and in counties with significant petroleum, chemical, paper, smelter, and shipbuilding industries.46,79–84 Studies of professional drivers who generally have a greater daily exposure to exhaust fumes than nonprofessional drives have shown an increased risk of lung cancer incidence and mortality in these individuals in most but not all studies.85–93 However, the role of air pollution as a cause of lung cancer in the urban and industrialized environments is a complex and controversial issue, and conclusions among various studies are conflicting.45,94–98
Cigarette consumption has been far greater in the past in the industrialized countries with higher lung cancer mortality rates than in nonindustrialized countries, and cigarette smoking may have been more prevalent in urban areas than in rural areas early in the 20th century, leading to the increases in lung cancer rates in industrialized and urban environments.39,99 Although smoking was accounted for in several studies of exhaust exposure in professional drivers, it is known that professional drivers smoke more than the reference population.92 The role of particular industries in increasing lung cancer risk in a given area may be limited to selected workers with specific occupational exposures such as asbestos in shipyard workers, although an increased risk for the general population due to exposure to air contamination from certain industries has been hypothesized in some cases.79,82,100 The contribution of air pollution to lung cancer in urban centers may be largely in combination with heavy smoking. Air pollution has been suggested to contribute to 10% of lung cancers in large cities.39 Vena101 estimated a fourfold increased risk factor of lung cancer in heavy smokers exposed to heavy air pollution compared with low air pollution.
Radon Gas
Radon gas was suspected of causing lung cancer in metal ore miners in Germany and Czechoslovakia in the early 20th century, and it has since been established as a cause of lung cancer for those who work in radon-contaminated uranium and other mines.102–104 Radon gas is also a common indoor environmental pollutant, which has raised the question of whether there is a link between presumed or measured household radon exposure and the development of lung cancer. This issue is highly controversial and unresolved at present. There are uncertainties in extrapolating data from miners to the general population. It has been suggested that the Environmental Protection Agency’s risk estimate that 20,017 lung cancer deaths annually in the United States can be attributed to indoor radon be reduced by ~25%.105–107 Several reviews of this topic have found the link between indoor radon and lung cancer to be weak and inconclusive.108,109 However, a recent meta-analysis found a potential effect of residential radon on the risk of lung cancer110 and another recent review suggests that indoor radon gas may contribute to 1% of lung cancers.111
Occupational Exposures
In the appropriate setting, exposure to specific occupational carcinogens, either alone or in combination with cigarette smoking, may increase the risk of lung cancer.112 As with cigarette smoking, there is a latency that may be up to 50 years between first exposure to the carcinogen and development of lung cancer,113 and there is generally a relationship of the amount and duration of exposure to the risk of developing lung cancer.99,114,115 Occupational agents that have been associated with an increased risk for lung cancer include asbestos fibers in the setting of asbestosis,116–119 coke,114 arsenic,120–122 chromium,123,124 nickel,125,126 chloromethyl ethers,127–129 vinyl chloride,130,131 and radon gas in miners.103,104 Increased risk of lung cancer has been reported in specific occupations including painters and nonferrous metal workers.132 Evidence for an increased risk with other occupational agents such as beryllium is limited.133
Attribution of a lung cancer to a specific occupational carcinogen is often complicated by exposure to a variety of potential agents or by a positive smoking history. Occupational carcinogens may contribute to ~15% of lung cancers in men and 5% in women.39
It has been suggested that most occupational carcinogens are associated with a selective increase in one or two cell types of lung cancer.134 However, limitations related to demographic variables, techniques in obtaining and preparing tissue specimens, sources of specimens, observer variability, and sources of information may produce artifactual associations of cell type and exposure.29 An increased percentage of small cell carcinomas has been reported in uranium miners exposed to radon gas135–138 and in workers exposed to chloromethyl ethers.87,113,114 However, other factors such as age of the worker may play a role in these apparent observations.139 As with cigarette smoking, an increase of all cell types is seen in lung cancers attributed to asbestosis83 and arsenic.29
The effects of occupational exposures combined with cigarette smoking are variable. Because former smokers continue to have a risk of developing lung cancer for many years that is greater than that of those who have never smoked, care must be taken in interpreting data that compare occupational exposures of current smokers to nonsmokers, because the latter group may include former smokers as well as lifelong nonsmokers. Chloromethyl ether and nickel apparently do not require combination with cigarette smoking to result in an increased risk of lung cancer.114 On the other hand, although there is a strong multiplicative or synergistic effect of cigarette smoking on the risk of lung cancer in workers with asbestos exposure (and specifically asbestosis when this issue is addressed), the relative increased risk of lung cancer among nonsmoking asbestos workers is small and has been suggested only on the basis of a surprisingly few reported cases.117–119,140,141 The epidemiologic findings combined with experimental studies have led to the hypothesis that asbestos is not a complete respiratory tract carcinogen or initiator of lung cancer, but rather a tumor promoter or cocarcinogen for lung cancer that interacts with other agents such as the compounds in cigarette smoke.112,142,143
Not all workers potentially exposed to the same occupational carcinogen share a uniform risk for the development of lung cancer. The dose to which a worker is exposed may depend on other factors in addition to simple duration of employment. For example, coke workers who work above the oven receive a greater dose than those working at the side of the oven and have an approximately three times greater risk of developing lung cancer.114
In the original study by Sir Richard Doll144 in 1955 showing excess risk of lung cancer in asbestos factory workers, all lung cancer cases had evidence of asbestosis, that is, diffuse interstitial fibrosis due to asbestos exposure. Since then, multiple studies have demonstrated that asbestos exposure does not increase the risk of lung cancer except in the setting of asbestosis.145–152 This observation means that the mere presence of asbestos bodies on histologic sections is not sufficient to attribute a lung cancer to asbestos exposure and that the pathologist must document the presence of asbestosis (fibrosis) in lung parenchyma uninvolved by tumor to make such an attribution.112,118,119,143
Scars and Diffuse Fibrosis
Peripheral carcinomas, particularly subpleural adenocarcinomas and especially bronchioloalveolar carcinomas, are frequently associated with a focal scar. In the most classic examples, the scar contains dense collagen and anthracotic pigment and results in puckering of the over-lying pleura. This has led to the hypothesis that many peripheral carcinomas arise in scars (so-called scar cancers). These scars may be the result of bronchiectasis,153,154 infarcts,155 tuberculosis,156 or trauma.157 Earlier studies have estimated that 3 to 7% of lung carcinomas are scar cancers.158,159 About three fourths of these were adenocarcinomas.159 It has been speculated that the atypical alveolar epithelium associated with focal scars is a precursor to the scar cancers.99
Although there is no doubt that some peripheral carcinomas are associated with undisputed preexisting scars, studies indicate that the focal fibrosis associated with most lung cancers formerly regarded as scar cancers is a desmoplastic reaction to the tumor similar to that seen in other primary tumors such as breast cancer.160–164 This latter conclusion is based in part on observations that the age of the scar correlates with the age of the tumor as suggested by the fact that the size of the scar corresponds with the size of the tumor,160 and the age of the scar as determined by cellularity and collagenization correlates with prognosis and, therefore, the duration of the tumor.162 In addition, studies of the scars have shown atelectasis of the parenchyma,163 increased amounts of type III collagen,161 and greatly increased numbers of myofibroblasts as compared with apical scars,164 which are further evidence that the fibrosis is a desmoplastic reaction rather than a preexisting scar in the cases examined. Therefore, radiographic, clinical, or histopathologic evidence (i.e., old granuloma, etc.) of a preexisting scar should be present before a lung cancer can be called a scar cancer with certainty. Given the prevalence of pulmonary scars in the general population, the percentage that actually gives rise to a carcinoma must be very small.162
An increased risk for the development of lung cancer has also been associated with the diffuse interstitial fibrosis and honeycombing seen with chronic interstitial lung diseases including idiopathic pulmonary fibrosis (cryptogenic fibrosing alveolitis or usual interstitial pneumonia),165–167 sarcoid,168 rheumatoid lung,169 and systemic sclerosis.170–172 The relationship of asbestosis, another form of diffuse interstitial fibrosis, to lung cancer was discussed earlier (see Occupational Exposures). In one series of 205 patients with idiopathic pulmonary fibrosis, ~10% developed lung cancer. The ratio of observed to expected lung cancers was increased (14.2 for male smokers and 6.7 for female smokers) even when smoking history was taken into account. No difference in the prevalence of cell types compared with lung cancers without preexisting fibrosis were noted.167 In a recent study from the Mayo Clinic, most patients with idiopathic pulmonary fibrosis and lung cancer were older male smokers with peripheral squamous cell carcinomas arising in the areas of scarring.173 The increased risk factor for lung cancer in patients with interstitial fibrosis due to systemic sclerosis has been reported to be 16.5.172 As with focal scars, the atypical epithelium associated with honeycombing has been speculated to be a precursor lesion to carcinoma.165–167,174,175 Stimulation of growth factors and activation of oncogenes leading to both fibroblast proliferation and epithelial proliferation may account for these associations.176
Hereditary and Socioeconomic Factors
Several studies have shown an association between a positive family history of lung cancer and an increased risk of lung cancer.177–181 However, an increased lung cancer risk in a family could also be due to the effects of passive smoking or shared smoking habits or occupations.65 Increased incidence rates among Chinese and a greater increase in incidence rates among blacks than whites have been reported,46,182,183 but the latter may reflect the fact that cigarette smoking is now more prevalent among blacks than whites.3 An apparent association of lower socioeconomic status with an increased risk of lung cancer183,184 is probably the result of the differences in smoking habits among socioeconomic, occupational, and educational groups.4,185
Nutritional Factors
The suggestion that β-carotene and retinoids may be useful as chemopreventive agents in patients at risk for lung cancer is controversial, and some studies have reported increased mortality with β-carotene supplementation.178,186,187 Studies have found a possible protective role for high dietary intake of fish, fruit, and vegetables.71,188–190 A diet rich in fruits and vegetables is proposed to reduce the incidence of lung cancer by ~25%.191
Human Immunodeficiency Virus
Lung cancer is uncommonly reported in patients who are seropositive for human immunodeficiency virus (HIV),192–194 but a recent study has reported an apparent increased prevalence of lung cancer in HIV-infected patients.195 Virtually all of the HIV-infected patients reported to have lung cancer are smokers (median of 60 pack years in one series193). Lung cancers in HIVseropositive patients are reported to occur in a younger population, mostly male, and to have a shorter median survival than in patients whose HIV status is indeterminant (not known). Because HIV-seropositive patients represent a younger population, mostly male, in the first place and have a shortened survival due to HIV-related diseases such as opportunistic infections anyway, it is debatable as to whether these apparent observations about lung cancer in these patients are anything more than coincidental.196
Carcinogenesis, Molecular Genetics, and Histogenesis of Lung Cancer
Carcinogenesis of lung cancer, as with other types of cancer, is a multistage phenomenon with accumulation of multiple independent molecular alterations that impact critical genetic pathways during the development of the cancer from normal epithelium. Carcinogens produce irreversible DNA mutations in previously normal bronchial or other epithelial cells, thus initiating carcinogenesis. Mutations result in loss of normal cell growth control and abnormalities in apoptosis or programmed cell death. Tobacco smoke contains a multitude of known and suspected carcinogens that contribute to the development of lung cancer. The additional occupational and environmental exposures mentioned earlier may also act as carcinogens or cocarcinogens in some cases. Metabolic activation of carcinogens by the exposed host may also influence the process of carcinogenesis.11,22,197–199
Some of the mutations produced by the carcinogens are biologically significant and contribute to the development or progression of the malignant phenotype. Mutations may modify the protein products of oncogenes that regulate cell growth. Other mutations modify the protein products of tumor suppressor genes that inhibit cell growth. The result, in simplified terms, is loss of control of cell growth and disruption of major positive and negative signal-transduction pathways that regulate cell growth.200 The actual process of carcinogenesis involves a very complex series of interactions between multiple genetic abnormalities.201–205 In addition to oncogenes and tumor suppressor gene abnormalities, growth factors and signaling molecules, microsatellite alterations, aberrant DNA methylation, telomerase activation, angiogenesis, and individual host susceptibility have roles in the development of lung cancer. Tumor acquired promoter hypermethylation is a mechanism for the epigenetic inactivation of gene expression. The hallmarks of cancer related to cellular and genetic abnormalities include (1) abnormalities in self-sufficiency of growth signals, (2) evasion of apoptosis, (3) loss of sensitivity to antigrowth signals, (4) unlimited replicative potential, (5) sustained angiogenesis, and (6) tissue invasion and metastases.206,207
Techniques used to investigate these various molecular abnormalities in lung cancer include proteomics and immunohistochemistry, fluorescence in situ hybridization (FISH), polymerase chain reaction (PCR) amplification, reverse-transcriptase polymerase chain reaction (RT-PCR) amplification and real-time PCR, comparative genomic hybridization (CGH), loss of heterozygosity (LOH), complementary DNA (cDNA), microarray analysis, DNA sequencing, and short or small interfering RNA (siRNA) with assistance from sampling methods like laser capture microdissection. Clinically manifest lung cancers accumulate many, probably 20 or more, clonal genetic and epigenetic abnormalities during the multistep process of their development. Complex pathways in cell cycle and growth control such as the p53/HDM2/p14ARF pathway and the p16-cyclin D1-CDK4-RB pathway permit more than one step at which alterations can disrupt the pathway and cause the same end result. Molecular genetic abnormalities thought to be important in lung cancers are listed in Table 17–4.200,207,208 Some of these alterations such as epidermal growth factor receptor (EGFR) and c-kit (CD117) are targets of experimental therapies.209–213
| Non–Small Cell (%) | Small Cell (%) |
|---|---|---|
p53 (mutation) | 50 | >75 |
p53 (expression by immunostain) | 40–60 | 40–70 |
17p/p53 (LOH) | 70 | 80–90 |
HDM2 (overexpression) | 25 |
|
Rb 1 (lost expression) | 15–30 | 90 |
13q/Rb 1 (LOH) | 40–60 | 75 |
Cyclin D1 (overexpression) | 25–47 |
|
p16INK4A (mutation) | 10–40 | <1 |
p16INK4A (lost expression by immunostain) | 30–70 | 0–10 |
9p/p16INK4A | 50–75 | 20–50 |
3p (allele loss) | 50–80 | >90 |
EGFR/ERBB1 (overexpression) | 30–50 |
|
HER2/neu/ERBB2 (overexpression) | 30 | <1% |
ERBB3 (high expression by immunostain) | 18.6 |
|
GRP loop | 20–60 |
|
CD117 | 13–17 | 78–82 |
KRAS (mutation) | 15–20 | <1 |
myc family (amplification) | 8–20 | 18–31 |
c-myc (amplification/overexpression) | 5–10 | 15–30 |
bcl2 expression | 10–35 | 75–95 |
Telomerase expression | 80–85 | 100 |
Other deletions 5q, 8p, 11p, 18q | Variable | Variable |
Modified from Fong KM, Sekido Y, Mina J. The molecular basis of lung carcinogenesis. In: Coleman WB, Tsongalis GJ, eds. The Molecular Basis of Human Cancer. Totowa, NJ: Humana Press, 2002:379–405; and based on Fong KM, Sekido Y, Gazdar AF, Minna JD. Lung cancer. 9: Molecular biology of lung cancer: clinical implications. Thorax 2003;58:892–900, and other references (see text).
Specific mutations may be associated with specific carcinogens. Point mutations in codon 12 of the K-ras oncogene, usually a guanine to thymine conversion, occur in 30% of adenocarcinomas from smokers but in only 5% of adenocarcinomas from nonsmokers.214,215 Compounds found in tobacco smoke such as benzo[a]pyrene induce similar K-ras mutations in lung tumors of laboratory animals.216–221 Mutations of K-ras codon 12 are distinctive for adenocarcinoma of the lung but are only rarely seen in other lung cancer cell types.214,222
Alterations of the p53 tumor suppressor gene are found in 75 to 100% of small cell lung cancers, 51% of squamous cell carcinomas, 54% of large cell carcinomas, and 39% of adenocarcinomas.223 In a study of primary pulmonary adenocarcinomas, p53 protein was detected by immunostaining in 56% of tumors from current smokers and 33% of tumors from former smokers but in none of the tumors from those who have never smoked, suggesting that the p53 gene is a target of specific mutagens in tobacco smoke.224 Benzo[a]pyrene, a carcinogen in tobacco smoke, selectively forms adducts at guanine positions in codons 157, 248, and 273 of the p53 gene, which are known to be major p53 gene mutational hot spots.225
Different patterns of abnormalities of the p53 suppressor gene occur in lung cancers from individuals whose only known risk factor is cigarette smoking compared with lung cancers from cigarette smoking uranium miners who have the additional risk factor of radon exposure. The most frequent base transversion in the p53 tumor suppressor gene in lung cancers from smokers is a G:C to T:A transversion and deletions are rare.226–229 In a study of 19 lung cancers from radon-exposed uranium miners, the G:C to T:A transversion was not found, and deletions of p53 were relatively common.230 Thus, when an additional carcinogen, radon gas, is added to the carcinogens present in cigarette smoke, the type of p53 mutations seen in lung cancers is altered.
As significant mutations accumulate due to exposure to carcinogens, there is a progression in biologic behavior and light microscopic features from normal epithelium to premalignant stages that, as genetic abnormalities accrue, become more and more phenotypically malignant. This process is best documented in the development of squamous cell carcinoma in which progressive premalignant lesions can be relatively easily detected.231,232 It has been proposed that small foci of atypical adenomatous hyperplasia may be the precursor lesions of peripheral adenocarcinomas.233–237 Presumably increasing cytologic atypia in association with increasing genetic abnormalities of the alveolar epithelium would progress to frank malignancy analogous to the premalignant metaplastic, dysplastic and carcinoma-in-situ lesions in squamous cell carcinoma (see later).
Genetic abnormalities precede the histopathologic changes. Increased expression of the c-myc protein product, p62c-myc, can be detected by immunostaining in the nonmalignant bronchial epithelium of resection margins from lung cancer specimens compared with the bronchial epithelium from control (lung transplant) specimens.238 This observation is consistent with a “field effect” of carcinogens on the bronchial epithelium and alveolar epithelium, which results in the risk of development of more than one primary lung cancer, a phenomenon that is reported in ~2% of cases overall and more frequently in patients surviving resection of their initial tumor.239–243
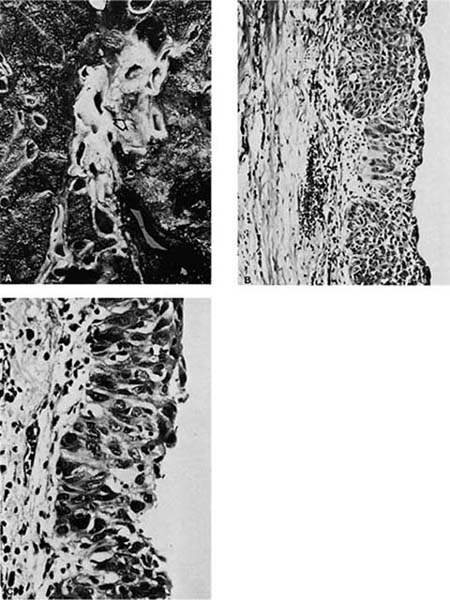
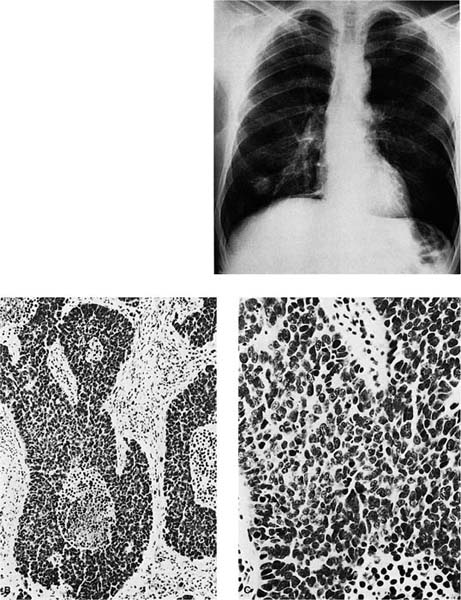
Sequential, increasingly severe cytologic changes have been documented in the development of squamous cell carcinoma in which there is a long progression from bland squamous metaplasia to mild to moderate to severe dysplasia to squamous cell carcinoma in situ and finally to invasive squamous cell carcinoma (Fig. 17–1).231,232 During the progression from normal epithelium to invasive carcinoma, cells show increasingly abnormal nuclear contours, increasing nuclear size, increasing nuclear-to-cytoplasmic ratio, and increasing hyperchromasia (which presumably reflects increasing DNA content). These increasing degrees of cytologic atypia of the bronchial epithelium presumably parallel increasingly numerous and increasingly significant genetic abnormalities within the successive generations of epithelial cells as the process of carcinogenesis progresses. In a study of bronchial mucosa specimens with progressively severe histopathologic changes ranging from normal to microinvasive carcinoma, Bennett et al244 did not detect mutant p53 protein in normal mucosa or squamous metaplasia. Increasingly severe degrees of atypia were associated with increasingly frequent detection of mutant p53 protein: 40% of mild dysplasias, 43% of moderate dysplasias, 90% of severe dysplasias, and 100% of carcinoma in situ and microinvasive carcinomas. Once the malignant phenotype has developed, there may be continued accumulation of genetic abnormalities, resulting in the development of new features such as an increasing tendency to metastasize. A period of many years typically passes between the initiating events and the clinical manifestation of the lung cancer due to the time elapsed between successive stages in the development of the malignant phenotype and the time required for the tumor to grow to a size that is detectable or produces symptoms. Detection of malignant cells on sputum cytology is often possible before a lesion is visible grossly or radiographically.245
FIGURE 17–1 Carcinoma in situ. (A) Gross appearance of lesion showing thickened bronchial mucosa (arrows). (B,C) Microscopic appearance of carcinoma in situ. Note the replacement of the epithelium by cytologically malignant cells that extend from the basement membrane through the surface. A possible focus of early invasion is present in B (B, ×160; C, ×400).
Although lung cancers can be divided into four primary cell types with generally distinctive histopathologic patterns and clinical correlates, it is likely that all lung cancers are ultimately derived from a normal epithelial cell, which, in addition to undergoing malignant transformation, can potentially differentiate into any of the cell types or into combined forms.246–249 This is in contrast to earlier theories that the different cell types reflected derivation from different types of specialized cells (i.e., small cell cancers from neuroendocrine cells, adenocarcinomas from goblet cells, etc.).246 In simple terms, the phenotype of the lung cancer, including not only the cell type or types but also other characteristics such as growth rate, metastatic potential, and response to particular forms of therapy, is believed to be the expression of the specific genetic abnormalities present. The development of more than one clone within the tumor would explain the histopathologic heterogeneity seen in lung cancers, including the differences in major histopathologic types seen within an individual tumor in up to 45% of sufficiently sampled lung cancers.250
These concepts are exemplified by the relationship association of increased myc expression in small cell lung cancer (SCLC) with the development of resistance to therapy and the apparent evolution of SCLC into non–small cell lung cancer (NSCLC). SCLC differs from NSCLC not only in its pathologic appearance but also in some aspects of its biologic behavior including its frequent neuroendocrine differentiation and temporary response to radiation and chemotherapy. Amplification of myc oncogenes is observed up to four times as often in SCLC from chemotherapy-treated patients as in SCLC from untreated patients.251 This suggests that treatment kills the chemotherapy-sensitive portion of the tumor, which lacks myc amplification, leaving behind chemotherapy-resistant clones with myc amplification resulting in a relapse of the tumor.
Furthermore, in vitro studies have shown that SCLC cell lines with increased c-myc expression have decreased neuroendocrine features and increased proliferative indices. The insertion of an activated H-ras oncogene into a SCLC cell line with an amplified myc oncogene causes the cell line to develop in vitro phenotypic features of NSCLC, including adherent monolayer growth patterns, ultrastructural features of NSCLC, development of NSCLC biochemical markers, and, importantly, resistance to chemotherapy and radiation therapy.252–254 In some cases, SCLC may show combined morphologic features of NSCLC in tissue specimens at the ultrastructural level, indicating that clones with NSCLC features may be present in vivo prior to therapy.255–260 These findings possibly explain the observation that NSCLC is often found at autopsy of patients with a previous biopsy diagnosis of SCLC.255,261–263 Therapy may select for a clone of cells with a therapy-resistant NSCLC phenotype that flourishes after the therapy-sensitive SCLC clones have died off. All of these observations are consistent with the hypothesis that all cell types are derived from a common precursor and that the phenotype of a particular lung cancer depends on the specific genetic abnormalities present and not on the features of an alleged specialized cell of origin.
Genetic abnormalities occurring with some frequency in the early stages of lung cancer development could prove useful in early detection on cytology specimens or in differentiating malignancy from reactive atypia on small biopsies. Mutant p53 protein identified by immunostaining is an example of a potential molecular marker in this category, although no marker has yet proven completely reliable. Variables include antibody used and retrieval methods, and others.264,265 An abnormality that occurs in association with the progression of lung cancers may be used as a marker to divide early-stage lung cancers into those likely to be more aggressive or metastasize (marker present) and those that are not (marker absent). Molecular markers for predicting prognosis in lung cancer are further discussed later (see Prognosis of Lung Cancer).
Heterogeneity of Cell Types
It is unusual for a lung cancer to consist of a uniform cell population. As already noted in the previous section, different major cell types, for example, combined small cell carcinoma and adenocarcinoma, may be identified within a single tumor in up to 45% of sufficiently sampled lung cancers.250 This observation is consistent with the concept that all lung cancers arise from cells with the potential to differentiate into any of the cell types. Clones of different cell types could arise within a tumor or be selected for by therapy as in the example of progression of SCLC to NSCLC.
As a practical matter, most lung cancers can be classified as one cell type or another and the presence of another cell type is usually not of major prognostic or therapeutic significance compared with such factors as stage of disease.266 If they are significantly affected at all by cell type heterogeneity, prognosis and treatment are likely to be most affected by the presence of a component with poor prognosis. Mixed small cell/large cell carcinomas have a 58% response rate to chemotherapy with a survival time of 6 months compared with a 91% response rate and 10.5-month median survival time for “pure” small cell carcinoma.267 Although this example shows a difference in clinical behavior due to cell type heterogeneity, the prognosis is still very poor for both groups.
Pathologic classification of the tumor should emphasize the major cell type and may note significant minor components, that is, “small cell carcinoma (90%) with adenocarcinoma component (10%).” When the components are roughly equal, designations such as adenosquamous carcinoma (i.e., 50% adenocarcinoma, 50% squamous cell carcinoma) may be appropriate. More frequently, a mixture of populations of the same basic cell type may be present, that is, moderately well differentiated acinar adenocarcinoma with a minor component of poorly differentiated solid adenocarcinoma. Determination of cell type heterogeneity, when it is present, is influenced by sampling and by the subjective interpretation of cell type by the pathologist. As noted in the previous section, in the future the molecular genetic profile may be a more important and more objective consideration than cell type in determining classification of lung cancers for prognosis and treatment.
Frequency of Cell Types
As noted in the introductory section of this chapter, lung cancer is divided into four major histologic cell types: small cell carcinoma, squamous cell carcinoma, adenocarcinoma, and large cell carcinoma. The histopathologic and other specific features of these cell types are discussed later. An overview of the frequency of these cell types is presented here. The prevalence of the cell types is influenced by sex and smoking history.
Historically, squamous cell carcinoma constituted the largest proportion of lung cancers, but in the United States there has been a relative increase in the incidence of adenocarcinoma over the past three decades and it is now the most prevalent cell type in many series.23,268–272 A similar trend has been reported in Japan.273 A general estimate of the proportion of each cell type is 35% for squamous cell carcinomas, 35% for adenocarcinomas, 15 to 20% for small cell carcinomas, and 15% for large cell carcinomas. The percentage of squamous cell carcinomas varies from 30 to 40%, for adenocarcinomas from 20 to 40%, for small cell carcinomas from about 10 to 25%, and for large cell carcinomas from 10 to 30%.27 In addition to the expected interobserver differences in diagnosis, the selection of cases may account for the variability in cell type prevalence in different series. The frequency of cell types differs when surgical and autopsy series are compared,274 when biopsies are compared with surgical resections,275,276 and when hospital-based studies are compared with population-based studies.27 These differences may reflect therapy-induced histologic changes in autopsy compared with surgical specimens, limited sampling of biopsies compared with resections or autopsies, and selection of patients in hospital-based studies compared with the population at large.99
Gender, Smoking, Age, and Cell Type
All of the major cell types of lung cancer are associated with smoking and all are seen in both sexes. In 1962 when there were marked differences in smoking habits between the sexes, Kreyberg divided lung cancers into two groups on the basis of general epidemiologic and histopathologic characteristics. Group I tumors consisted of squamous cell and small cell carcinomas, which were strongly associated with smoking, seen predominantly in men, and tended to be centrally located in the lungs. Group II tumors consisted of adenocarcinomas and large cell carcinomas, which were more weakly associated with smoking, had a roughly equal sex distribution, and tended to be peripherally located in the lungs.277,278 Although the Kreyberg groupings are less often referred to today, most studies continue to show differences in the frequency of the major cell types according to the sex and smoking history.
In most series, adenocarcinoma is the most frequent cell type in women smokers and in nonsmokers of both sexes. Among nonsmokers with lung cancer, adenocarcinomas may account for as many as two thirds to three fourths of lung cancers in women and as many as one half of those in men.23,279–283 Although there appears to be a stronger relationship between smoking and squamous cell and small cell carcinomas, the great majority (~90%) of adenocarcinomas occur in patients with significant smoking histories.23,284,285 Among smokers with lung cancer, squamous cell carcinoma is generally the most frequently reported cell type in men and adenocarcinoma is generally the most frequently reported cell type in women, but some series indicate changing cell type prevalence among smokers. In the United States, multiple series have shown an increase in the prevalence of adenocarcinoma that is predominantly due to an increased prevalence of adenocarcinoma among men. It has been proposed that the increased prevalence of adenocarcinoma may be related to the use of filter cigarettes.286
In many of the same series a relatively increased prevalence of squamous cell and/or small cell carcinoma among women is reported, possibly due to changing smoking habits.23,27,272,287–291 In some series, overall prevalence of squamous cell carcinoma has been reported to be increasing.292 In the past, some reports have suggested a greater proportion of squamous cell carcinomas among older patients and a relatively greater proportion of adenocarcinoma and small cell carcinoma among younger patients.284,293,294
Differences in cell type prevalence between the sexes may represent differences in smoking habits or host-related factors such as hormonal mileu.295 The differences in cell type prevalence between smokers and nonsmokers may result from different genotypic alterations involved in the initiation and progression of malignancy in the two groups. The causes of these reported changes in cell type prevalence may reflect changes in smoking habits, changes in cigarettes (filtered versus unfiltered, lower tar), or changes in environmental pollution. The selection of cases in hospital-based studies versus population-based studies may also account for some of the apparent cell type discrepancies in reported series.27
Multiple Primary Lung Cancers
Two or more primary lung cancers may occur in the same patient either simultaneously (synchronous tumors) or at different times (metachronous tumors).239–243,296,297 Overall, multiple lung primaries occur in ~2% of all lung cancer patients. This relatively low percentage is perhaps due to the poor survival rate of lung cancer patients that precludes the clinical manifestation of a second primary in most cases. Patients who survive resection of one lung cancer have a higher incidence, as high as 10 to 32% in some series, of developing a second lung primary.296,297
About two thirds of the reported multiple lung primaries are metachronous.296,297 Even when two cancers are metachronous, it may be difficult to determine if a second tumor represents a second primary or a metastasis from the original tumor. General criteria for determining that two lung cancers are separate primaries are listed in Table 17–5. Differences in histology, particularly different cell types, may be helpful but are not absolute because, as already noted, lung cancers are frequently heterogeneous. Multiple primaries may also be of the same cell type. Differences in immunostaining patterns, including immunostaining for oncogene or tumor suppressor gene products, should be interpreted with care because different clones of the same tumor may not express the same proteins, and this includes metastases compared with the primary tumor. When a patient has two (synchronous or metachronous) lung primaries, the tumors should be staged separately. Generally, treatment and prognosis of separate primaries is determined independently for each tumor and depends on the stage and other independent factors of each primary. However, loss of functional lung tissue due to previous resection or other therapy may influence the approach to any given case.
Cancers are separate in location (synchronous and metachronous) and/or time (metachronous) |
Histology is different |
Histology is the same and: |
Separate origin from carcinoma in situ is identified |
No cancer is identified in lymphatics shared by both tumors |
No extrapulmonary metastases are present at the time of diagnosis |
Based on Martini N, Beattie EJ, Cliffton EE, Melamed MR. Radiologically occult lung cancer—report of 26 cases. Surg Clin North Am 1974;54:811–823; Carter D. Pathology of early squamous cell carcinoma of the lung. Pathol Ann 1978;13(pt 1):131–147; Bower SL, Choplin RH, Muss HB. Multiple primary bronchogenic carcinomas of the lung. AJR 1983;140:253–258; and Bewtra C. Multiple primary bronchogenic carcinomas, with a review of the literature. J Surg Oncol 1984;25:207–213.
Pathology of Specific Cell Types (Table 17–6)
Squamous Cell Carcinoma
Squamous cell carcinoma, also called epidermoid carcinoma because of its histopathologic resemblance to epidermis, is characterized histopathologically by relatively large cells that typically have relatively abundant cytoplasm. The diagnostic features of squamous cell carcinoma are formation of intracytoplasmic keratin, which may result in the formation of keratin pearls, or the presence of intercellular bridges (“prickles” between cells). Although the World Health Organization (WHO) classification defines squamous cell carcinoma using these criteria, the ease with which these features are identified is dependent on the degree of differentiation of the tumor.12,298–301
Squamous cell carcinoma |
Variants: |
Papillary |
Clear cell |
Small cell |
Basaloid |
Small cell carcinoma |
Variant: |
Combined small cell carcinoma |
Adenocarcinoma |
Adenocarcinoma with mixed patterns |
Acinar adenocarcinoma |
Papillary adenocarcinoma |
Bronchioloalveolar |
Nonmucinous |
Mucinous |
Mixed nonmucinous and mucinous or indeterminate |
Solid adenocarcinoma with mucin production |
Variants: |
Fetal adenocarcinoma |
Mucinous (“colloid”) carcinoma |
Mucinous cystadenocarcinoma |
Signet ring adenocarcinoma |
Clear cell adenocarcinoma |
Large cell carcinoma |
Large cell neuroendocrine carcinoma |
Combined large cell neuroendocrine carcinoma |
Basaloid carcinoma |
Lymphoepithelioma-like carcinoma |
Clear cell carcinoma |
Large cell carcinoma with rhabdoid phenotype |
Adenosquamous carcinoma |
Carcinoid |
Typical carcinoid |
Atypical carcinoid |
Based on Travis WD, Brambilla E, Harris CC, Muller-Hermelink HK, eds. World Health Organization Classification of Tumours, Pathology and Genetics: Tumours of the Lung, Pleura, Thymus and Heart. Zurich: IARC, in press.
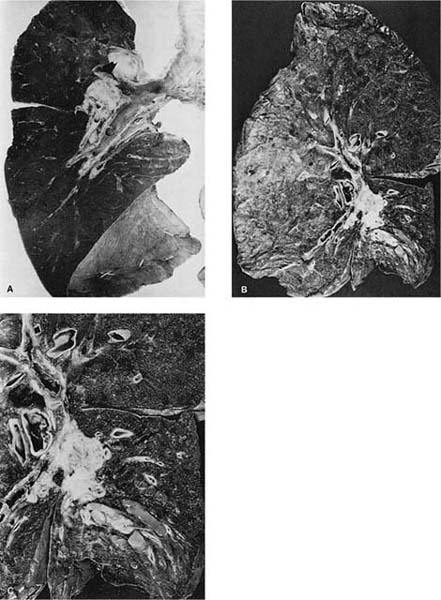
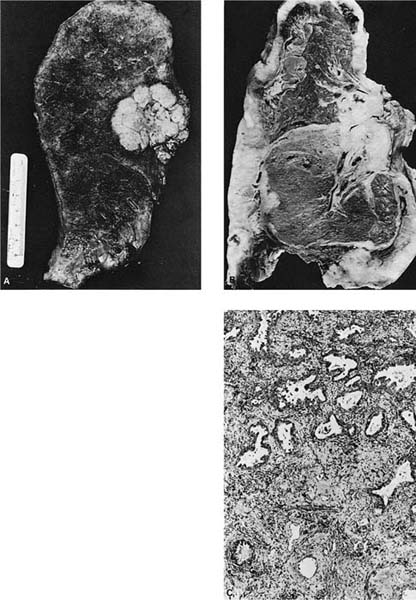
Grossly, most squamous cell carcinomas arise centrally in the lungs primarily in the main, lobar, segmental, or subsegmental bronchi (Fig. 17–2A–C),302,303 although as many as one third are reported to be peripheral in some series.7 Recently, Funai et al304 have divided peripheral squamous cell carcinomas into alveolar space-filling, expanding, and combined types. Squamous cell carcinomas are usually firm, off-white masses that protrude into the lumen of the involved bronchus, and invade the underlying wall and parenchyma, frequently extending proximally along the bronchus to the hilar lymph nodes. A common gross finding, especially in larger tumors, is central softening or cavitation of the tumor due to necrosis and/or keratinous debris.
Well-differentiated tumors show obvious or relatively abundant keratinization and intercellular bridges (Fig. 17–2D,E). The cells are typically arranged in cohesive nests or cords in a stratified arrangement reminiscent of epidermis or squamous mucosa. The cytoplasm is typically abundant and eosinophilic. Moderately differentiated tumors retain a nesting, stratified appearance but show fewer of the specific squamous features that may be focal within the tumor. Poorly differentiated tumors tend to form sheets rather than nests, and keratinization and intercellular bridges may be very focal and difficult to identify (Fig. 17–2F,G). Anaplastic cytologic features of the malignant cells and atypical mitoses tend to become more prominent as the degree of differentiation becomes less. In the most poorly differentiated tumors light microscopic features of squamous differentiation often cannot be distinguished, and these tumors are usually classified as large cell carcinomas or poorly differentiated non–small cell carcinomas, the latter in small biopsy samples.
These features of differentiation represent a continuous spectrum from well to poorly differentiated, and classification of a tumor into one of these subcategories has a significant subjective component, particularly in moderately and poorly differentiated tumors. Many tumors show varying degrees of differentiation within the same tumor.
Tumor necrosis is a common feature of many squamous cell carcinomas. The malignant cells, particularly in poorly differentiated tumors, may show clear cytoplasm due to glycogen. In some cases, tumor giant cells may be present especially after radiation therapy.
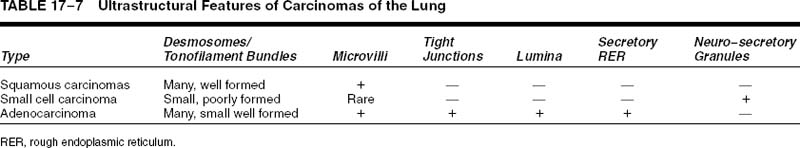
Squamous cell carcinomas are typically immunoreactive for keratins (AE1/AE3, CK5/6, and others), epithelial membrane antigen, and thrombomodulin. They are often immunoreactive for carcinoembryonic antigen, B72.3, MOC-31, and involucrin, which does not distinguish them from most other primary or metastatic lung carcinomas.305 Histochemical stains for keratin (Kreyberg stain) should be variably positive but are often difficult to interpret.278 Ultrastructurally, squamous cell carcinomas show intracytoplasmic tonofilaments and desmosomes and the intercellular bridges, which consist of cytoplasmic processes of two adjacent cells connected by a well-formed desmosome (Fig. 17–2H–K; Table 17–7). These ultrastructural features are relatively abundant in well-differentiated tumors and are sparse in poorly differentiated tumors.256,25,306–308
Rare histopathologic variants of squamous cell carcinoma consist partly or entirely of malignant spindle cells that must be distinguished from sarcomas and sarcomatoid mesothelioma. These tumors are currently classified under sarcomatoid carcinomas. Areas of typical squamous cancer are often present to confirm the diagnosis, although limited sampling on a small biopsy may create diagnostic difficulties. The malignant spindle cells are positive for keratin on immunostaining in most cases, but a negative keratin stain in the spindle cells does not exclude the diagnosis.12
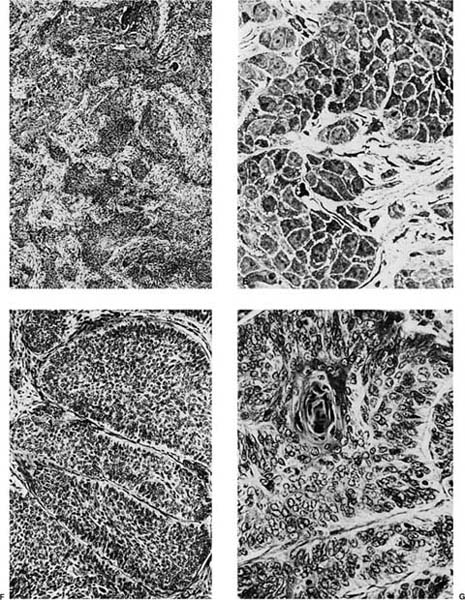
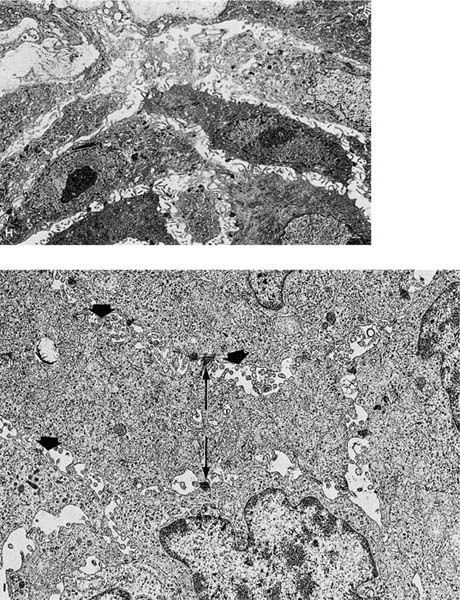
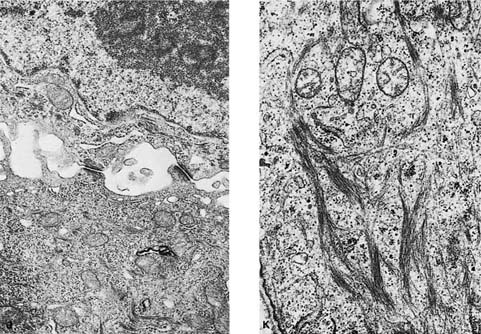
FIGURE 17–2 Squamous carcinoma. (A) Gross appearance of a central tumor arising in the main upper lobe bronchus (arrow). The tumor has already spread to hilar lymph nodes. (B,C) Squamous carcinoma arising in the lower lobe bronchus. The lower lobe is partially collapsed, and purulent bronchiectasis is present behind the tumor. (D,E) Moderately well-differentiated squamous carcinoma showing the diagnostic features of keratinization and formation of intercellular bridges or prickles (D, × 64; E, × 400). (F,G) Poorly differentiated squamous carcinoma. The bulk of the tumor appears similar to F, but rare foci of keratinization are present, as shown in G. Although some pathologists would classify this as a squamous carcinoma simply on the appearance in field F, strictly speaking one needs to find either intercellular bridges or evidence of keratinization, such as is shown in G (F, × 160 G, × 400). (H,I) Electron micrographs of squamous carcinoma. (H) A well-differentiated squamous carcinoma at low power. (I) A poorly differentiated squamous carcinoma at low power. Note that despite the light microscopy differences, both tumors show the defining ultrastructural features of numerous well-formed desmosomes and intercellular bridges (long arrows), as well as the formation of so-called interfacial canals (short arrows), spaces that mimic lumina. These spaces are not, however, bounded by tight junctions but by well-formed desmosomes (H, ×2017; I, ×10,017). (J,K) The outpouchings of cytoplasm connected by desmosomes as shown in J constitute the intercellular bridges. (K) Numerous tonofilament bundles, another characteristic ultrastructural feature of squamous carcinoma. (From Churg A. The fine structure of large cell undifferentiated carcinoma of the lung: evidence for its relation to squamous cell carcinomas and adenocarcinomas. Hum Pathol 1978;9:143–156 with permission.)
Rare tumors classified currently as variants of squamous cell carcinoma include papillary squamous cell carcinoma, small cell squamous cell carcinoma, and clear cell squamous cell carcinoma. Basaloid carcinoma is classified with squamous cell carcinoma when a squamous component is also present (the pure form is classified as a variant of large cell carcinoma).12 There are no apparent differences in biologic behavior or prognosis between the latter two variants and typical squamous cell carcinomas of the lung.309 Cancers arising in the apex of the lung (Pancoast’s tumors) are classically squamous cell carcinomas, most of which are large and cavitated (Fig. 17–3A,B).310 So-called small cell squamous cell carcinomas255 are rare, poorly differentiated tumors that have light microscopic features resembling small cell carcinoma (Fig. 17–4A–C) but have coarse or vesicular chromatin (as opposed to finely stippled “salt and pepper” chromatin), more conspicuous nucleoli, and more cytoplasm than true small cell carcinomas. They also have ultrastructural features of squamous cell carcinoma (Fig. 17–4D,E).
Adenocarcinomas
The diagnostic features of adenocarcinomas are gland formation and mucin production. The major histopathologic subtypes of adenocarcinoma include acinar (gland-forming), papillary, bronchioloalveolar carcinoma, solid adenocarcinoma with mucin, and adenocarcinoma with mixed subtypes.12 Because most adenocarcinomas are composed of two or more of the subtypes, most fall into the mixed subtype category. In many adenocarcinomas, there is a focal bronchioloalveolar-like pattern at the periphery of the tumor.284,298,301,305 Currently, bronchioloalveolar carcinoma is considered by some to represent carcinoma in situ. It is discussed separately because of its unique clinicopathologic features.12 Several variants of adenocarcinoma are recognized: fetal adenocarcinoma, mucinous (“colloid”) carcinoma, mucinous cystadenocarcinoma, signet ring adenocarcinoma, and clear cell adenocarcinoma.12
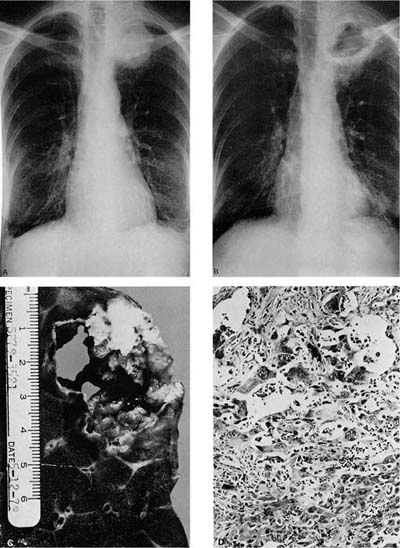
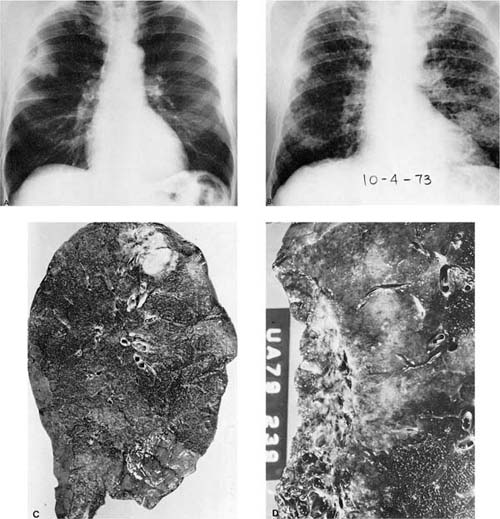
FIGURE 17–3 Pancoast’s tumor. (A,B) Chest radiographs showing a patient with a Pancoast’s tumor before and after radiation. (C) Gross appearance of the same tumor. The center of the tumor mass has become completely necrotic and has liquefied and disappeared. (D) Radiation change in the same tumor. The peculiar giant cells produced by radiation mimic the appearance of so-called giant cell carcinoma of the lung but are a common finding in any radiated adenocarcinoma or squamous carcinoma (×400).
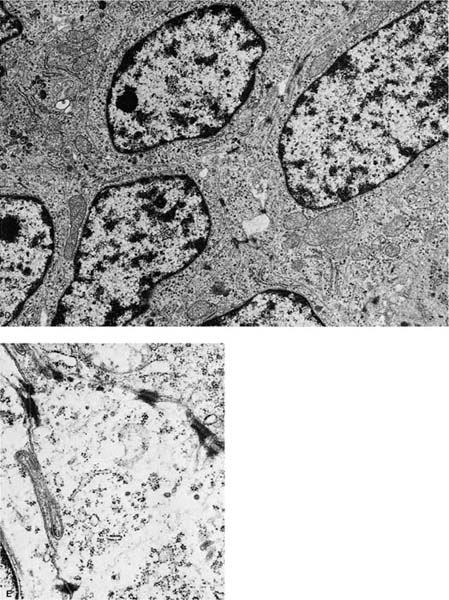
FIGURE 17–4 Small cell squamous carcinoma. (A) Chest radiograph showing a right lower lobe coin lesion found incidentally in an asymptomatic male smoker. (B,C) Microscopic appearance of the lesion shown in A. By light microscopy this tumor superficially resembles a small cell carcinoma (B, × 160; C, × 400). (D,E) Electron micrographs of the same tumor showing cells connected by numerous well-formed desmosomes but no neurosecretory granules. A diagnosis of small cell squamous carcinoma was made. The patient survived for 21/2 years with no further therapy before recurrence of the tumor at the resection margin (D, ×6017; E, ×16,017). (From Fong KM, Sekido Y, Mina J. The molecular basis of lung carcinogenesis. In: Coleman WB, Tsongalis GJ, eds. The Molecular Basis of Human Cancer. Totowa, NJ: Humana Press, 2002:379–405 with permission.)
Classically, adenocarcinomas arise in the periphery of the lung, but they may also arise centrally in a bronchus (Fig. 17–5A). Tumor necrosis may occur in adenocarcinomas, but cavitation is rare. As noted previously, they are the most common cell type associated with peripheral scar cancers. They are usually firm but may be soft and mucinous depending on their histopathologic composition and the presence of desmoplasia or a scar. Growth beneath or into the visceral pleura produces a pucker on the pleural surface. Occasionally the tumor may extend out over the pleura and encase the lung mimicking the growth pattern of a mesothelioma (Fig. 17–5B). These pseudomesotheliomatous carcinomas are discussed later.
The characteristic feature of adenocarcinomas is the formation of glands with malignant cells arranged around a central lumen (Fig. 17–5C) or the production of mucin. The glandular lumens may vary in size and shape and are often surrounded by desmoplastic stroma or in some cases may infiltrate a preexisting scar. Holes created by the necrosis of cells in a squamous cell or large cell carcinoma and rosettes formed in small cell carcinomas or carcinoids should be distinguished from true glands in an adenocarcinoma. When present, solid areas typically consist of nests, cords, or sheets of large, polygonal cells with relatively abundant lightly eosinophilic cytoplasm and vesicular nuclei with prominent nucleoli. At least five mucin-positive cells in two high-power fields (HPFs) are recommended for classification as a solid adenocarcinoma with mucin by current WHO criteria.12 Lepidic growth of the tumor along intact normal or fibrotic alveolar septa in a bronchioloalveolar carcinoma-like pattern may be seen at the margins of a tumor that is otherwise predominantly acinar or acinar mixed with other patterns. Papillary adenocarcinomas have secondary and tertiary papillary structures that obliterate the underlying lung architecture, in contrast to bronchioloalveolar carcinomas that may sometimes have simple papillary structures within intact alveolar spaces. Micropapillary adenocarcinomas have simple papillary tufts without fibrovascular cores, and this feature is believed to be associated with a more unfavorable prognosis.12,311
Periodic acid-Schiff (PAS) with diastase, mucicarmine, or Alcian blue stains may show mucin within the central lumina of the glands or as intracytoplasmic droplets in the malignant cells. Some cells may have distinct intracytoplasmic vacuoles on routine hematoxylin and eosin stain that may stain positive for mucin. However, mucin stains are negative in about one third of adenocarcinomas.
Well-differentiated tumors show abundant formation of glands or acini, whereas moderately and poorly differentiated tumors show relatively more solid areas of tumor and fewer glandular structures. The most poorly differentiated tumors are composed of solid nests or sheets of malignant cells that avoid classification as large cell carcinoma or poorly differentiated nonsmall cell carcinoma only by the demonstration of intracytoplasmic mucin on mucin stain. The degree of cytologic atypia is usually greater in the poorly differentiated tumors.
Variants of adenocarcinoma are recognized. As with squamous cell carcinomas, adenocarcinomas may be composed of cells with clear cytoplasm due to intracytoplasmic glycogen and may have tumor giant cells.312 Clear cell adenocarcinomas should be distinguished from metastatic renal cell carcinomas. The latter is likely to be positive for fat on Oil Red O or similar stains. Mucinous or colloid adenocarcinomas consist of limited numbers of strips of tumor cells and individual tumor cells, often very well differentiated, floating in pools of mucin (see Chapter 18). Mucinous cystadenocarcinomas consist of mucin-filled cysts lined by a layer of tumor cells that typically exhibit apical mucin. Signet ring adenocarcinoma consists of tumor cells with large cytoplasmic mucin vacuoles that push the nuclei to one side resembling a signet ring (see Chapter 18). Fetal adenocarcinomas consist of tubules lined by glycogen-containing columnar cells that resemble fetal lung. The well-differentiated fetal adenocarcinomas were previously classified with the pulmonary blastomas because the epithelial component of pulmonary blastomas has the same histopathologic pattern as well-differentiated fetal adenocarcinoma. High-grade fetal adenocarcinomas are now recognized.12
Adenocarcinomas may be biphasic with both an epithelial acinar component and a spindle cell component that may share some of the immunostaining properties of the acinar component, particularly keratin immunopositivity, although this is not true in every case.313 These tumors are currently classified as pleomorphic carcinomas under the broader heading of sarcomatoid carcinomas.12
Like other lung carcinomas, adenocarcinomas are positive for keratin and epithelial membrane antigen on immunohistochemical staining. Several other immunohistochemical markers are positive in adenocarcinomas of the lung. A review of 30 published series of immunostaining patterns in pulmonary adenocarcinomas by Brown et al314 disclosed the following combined results for positive immunoreactivity: carcinoembryonic antigen (CEA), 86%; B72.3, 88%; Leu M-1 (CD15), 79%; secretory component, 68%; carcinoma antigen-125, 70%; vimentin, 18%; and thrombomodulin, 8%. Their study of 103 additional adenocarcinomas showed very similar results except that 15% of their tumors were positive for Ca-125 and 58% were reactive for thrombomodulin. Approximately 50% of moderately to well-differentiated adenocarcinomas react with antibody to Leu-7,315 ~50% of adenocarcinomas react with antibody to surfactant apoprotein,316,317 and ~75% of adenocarcinomas are reactive for thyroid transcription factor-1 (TTF-1).318 MOC-31 is immunoreactive in the great majority of pulmonary adenocarcinomas.319,320
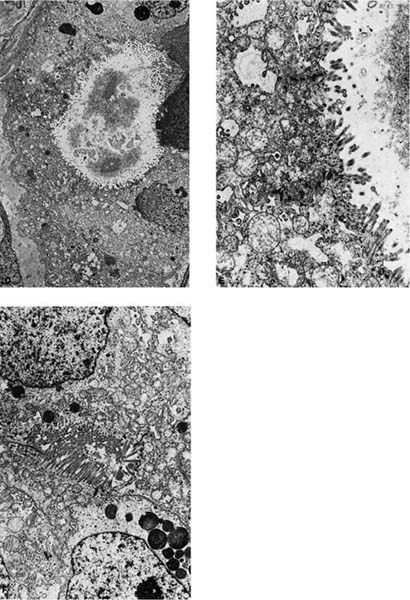
FIGURE 17–5 Adenocarcinoma. (A) Peripheral adenocarcinoma. This is the usual gross appearance of this type of tumor. (B) So-called pseudomesotheliomatous form of adenocarcinoma. The tumor has spread around the periphery of the lung in a fashion mimicking malignant mesothelioma. (C) Moderately well-differentiated adenocarcinoma showing gland formation. The tumor does not respect preexisting pulmonary architecture (×64). (D–F) Electron micrograph showing a formation of an intercellular lumen. This structure, which is bounded by junctional complexes (shown at higher power in E), is the ultrastructural prerequisite for the definition of a tumor as an adenocarcinoma (D, ×2017; E, ×10,017). (F) Electron micrograph of another poorly differentiated adenocarcinoma of the lung showing formation of an intercellular lumen. Note the long microvilli. This morphologic appearance casts doubt on the theory that mesotheliomas have longer and thinner microvilli than adenocarcinomas of the lung.
Ultrastructurally, adenocarcinomas show features of secretory cells that generally parallel the degree of differentiation. True lumina consist of inter- or intracellular spaces bounded by tight junctions and usually having a rim of microvilli that should be distinguished from microvilli-lined interfacial canals, which lack tight junctions and are found in squamous cell carcinomas. Secretory product is often present and may consist of flocculent-type granules, Clara cell-type granules, or type II cell granules, although the manner of fixation probably significantly influences the appearance of the secretory product. Other ultrastructural features include well-formed desmosomes, short tonofilament bundles, and rough endoplasmic reticulum (Fig. 17–5D–F).246,256,307
Bronchioloalveolar Carcinomas
Bronchioloalveolar carcinomas (BACAs) are a subset of well-differentiated pulmonary adenocarcinomas characterized histopathologically by lepidic (like a butterfly resting on a branch) growth of the malignant cells along intact alveolar septa that may be otherwise normal or may be mildly desmoplastic while retaining the original architectural pattern.321–324 This same pattern of growth may be mimicked by metastatic adenocarcinomas or may be present as a peripheral component of a primary lung adenocarcinoma that otherwise shows an acinar pattern. The designation “bronchioloalveolar carcinoma” was first used by Liebow321 to emphasize the apparent origin of these tumors from the bronchiolar and alveolar epithelium in contrast to the bronchial epithelium. Although some earlier authors questioned the distinction of BACA from other pulmonary adenocarcinomas,325–328 if strict definitions are used, the morphologic and clinical characteristics of these tumors are unique enough to distinguish them from other types of pulmonary adenocarcinoma.329,330 Currently, the WHO classification considers BACA to be a carcinoma in situ that does not exhibit invasion into stromal, vascular, or pleural tissues. Using this strict definition, resection of localized BACA results in a 5-year survival of 100%. There are reports that small adenocarcinomas with limited stromal invasion also have an excellent prognosis similar to purely in-situ BACA.12
| Nonmucinous | Mucinous |
|---|---|---|
Gross features | Usually solitary | Relatively frequent |
Multifocality | Not present | Occasional |
Pneumonic pattern |
|
|
Light microscopy | Not present | Present |
Apical mucin | Present | Not present |
Septal fibrosis | ⅓ | ⅔ |
Aerogenous spread |
|
|
Ultrastructure | 90% | — |
Clara cell | 33% | — |
Type II cell | — | 100% |
Mucin cells |
|
|
Based on Clayton F. The spectrum and significance of bronchioloalveolar carcinomas. Pathol Ann 1988;23:361–394; and Axiotis CA, Jennings TA. Observations on bronchiolo-alveolar carcinomas with special emphasis on localized lesions: a clinicopathological, ultrastructural, and immunohistochemical study of 11 cases. Am J Surg Pathol 1988;12:918–931.
BACA may be further subdivided into at least two types, mucinous and nonmucinous. Although both types share many pathologic features characteristic of BACA, there is a difference between the two types in the relative frequency of some of these features and hence prognosis (Table 17–8).329–331
In addition to the defining lepidic growth pattern, several other observations are associated with BACA. Although not seen in all cases, aeroginous spread (spread through the airspaces) is another well-known feature of BACA and is seen more frequently in the mucinous tumors.330 Traditionally, the BACA pattern is frequently seen at the periphery of small subpleural scar cancers,162 and a BACA pattern is the most frequently reported cell type of lung cancers in nonsmokers, although the majority of BACAs are associated with smoking.134,282,332 Although most BACA present as solitary nodules (Fig. 17–6A–C), a minority, particularly the mucinous type, present as widespread, bilateral, synchronous, multifocal nodules (Fig. 17–6D,E).333 In such cases, the prognosis is poor, and the “in situ” conceptualization breaks down somewhat. The multifocality of BACA is probably due to aeroginous spread in most cases, but may also be due to the simultaneous development of more than one primary.234,331 Jaagsiekte, a virus-induced disease in South African sheep, resembles the multifocal form of human BACA.334
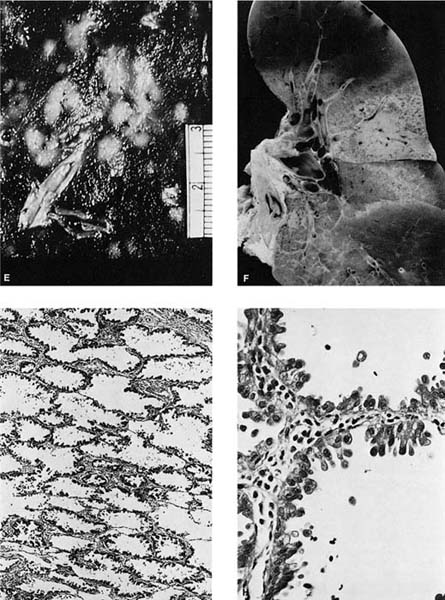
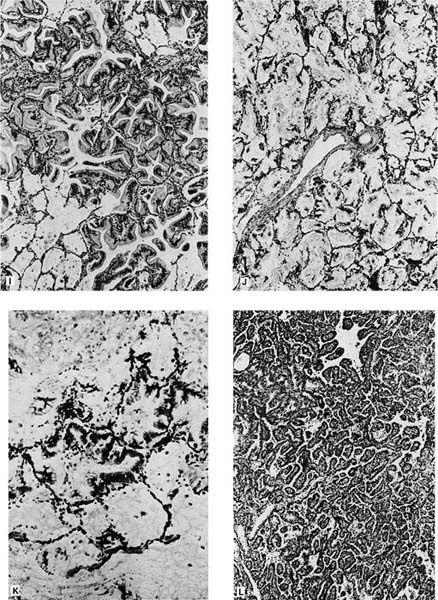
FIGURE 17–6 Bronchioloalveolar carcinoma. (A) Chest radiograph of a patient with bronchioloalveolar carcinoma presenting as a localized subpleural mass. (B) Chest radiograph of a patient with bronchioloalveolar tumor presenting as multiple bilateral nodules. (C) Gross appearance of bronchioloalveolar carcinoma presenting as a solitary nodule. (D) Gross of bronchioloalveolar carcinoma appearing as a poorly defined infiltrate. The adjacent lung has areas of honeycombing. (E) Gross appearance of bronchioloalveolar carcinoma presenting as multiple tumor nodules, corresponding to the chest radiograph shown in D. (F) Gross appearance of bronchioloalveolar carcinoma presenting in its so-called pneumonic form. No distinct tumor mass is seen, but the involved area of lung appears lighter and homogeneous. (G,H) Light micrograph showing growth of bronchioloalveolar carcinoma in typical lepidic fashion along alveolar walls. The higher-power view shows the peglike cells often found in the nonmucinous (G, × 64; H, × 400). (I) Mucinous type of bronchioloalveolar carcinoma (×64). (J,K) Pneumonic spread with diffuse filling of alveolar spaces by mucin and relatively scanty tumor cells along alveolar septa. (L) Simple papillary structures in a nonmucinous bronchioloalveolar carcinoma.
The nonmucinous type of BACA shows Clara cell/type II pneumocyte differentiation,316,322,335–340 and the mucinous type shows metaplastic bronchiolar mucous cell differentiation.331,333,341–343 A few tumors show mixtures of nonmucinous and mucinous cells. The so-called sclerosing BACAs are usually nonmucinous tumors with stromal fibrosis and should be distinguished from early invasive tumors.329,330,344
BACAs are usually found in the periphery of the lung and are often subpleural, although as many as 10% may be central in location.330 Grossly, BACAs may be firm if associated with stromal sclerosis or mucinous if associated with significant mucin production. When the underlying interstitium has little or no stromal response, the tumor may be soft, and tan in color to virtually indiscernible by sight or palpation. As already noted, tumors may be solitary or multiple. Occasionally, the mucinous type may present as a “pneumonic pattern” with consolidation of the alveoli by mucin producing a diffuse poorly circumscribed mucoid infiltrate (Fig. 17–6F). Subpleural tumors, particularly the sclerosing type, often result in puckering of the overlying pleura.
Histologically, the nonmucinous type consists of cuboidal to columnar cells with hyperchromatic nuclei or vesicular nuclei with prominent nucleoli lining intact alveolar septa, which frequently show mild to moderate interstitial fibrosis and chronic inflammatory infiltrate. The tumor cells often have apical snouts of cytoplasm and “hobnail cells” in which the columnar cells have a narrow base and apical hyperchromatic nuclei may be prominent (Fig. 17–6G,H). Eosinophilic intranuclear inclusions that are PAS positive and immunoreactive for surfactant apoprotein are often present.316 PAS with diastase stain may demonstrate scanty amounts of intracytoplasmic or intraalveolar mucin. Ultrastructurally, 90% of the nonmucinous tumors have cells with features of Clara cells such as electron-dense granules. About one third of the nonmucinous tumors have cells with ultrastructural features of type II pneumocytes such as lamellar bodies, in many cases in tumors that also have cells with Clara cell features.
The sclerosing BACA is characterized by central stromal sclerosis.160,162 The cells in the sclerosing type have light microscopic and ultrastructural features similar to those of cells of the nonmucinous type, and, therefore, the sclerosing type may be thought of as a variant of the nonmucinous type.330 Because the acinar type of pulmonary adenocarcinoma may have lepidic growth of tumor at its margins, it may be difficult to differentiate a sclerosing BACA without invasion from an acinar adenocarcinoma with invasion and peripheral lepidic growth. The presence of glands, desmoplastic reaction, and increased cytologic atypia favor invasion.12
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree