Carcinoma of the Esophagus
Jasmine Huang
Mohammad Bashir
Mark D. Iannettoni
The rapid growth in the incidence if esophageal cancer has resulted from many factors, such as increasing obesity and reflux, but probably the most important is the increased awareness on the part of the medical community as well as the patient and consumer. Many features of esophageal neoplasms have remained constant for decades: most tumors are malignant, and the usual presenting symptom is dysphagia. Although surgical resection offers the best chance for cure, most patients who present with symptoms already have systemic disease and are incurable with surgery alone. There are, however, other important new issues to consider, such as palliation of symptoms and quality of life issues related to swallowing. Esophageal cancer is no longer
synonymous with squamous cell carcinoma. Adenocarcinoma of the esophagus and the gastroesophageal junction are now more frequent in the United States and represent the fastest-growing solid malignancy. According to the American Cancer Society, the estimated incidence of esophageal cancer in the United States for 2008 was 16,470, with approximately 14,280 cancer-related deaths.11 Worldwide the total number of new cases of esophageal cancer was estimated at 500,000.158 The most important prognostic factor for esophageal cancer remains the stage of disease at the time of diagnosis. Currently, the best strategy for improving survival is early diagnosis. As the molecular biology of esophageal cancer is better understood, novel diagnostic, therapeutic, and prognostic modalities continue to be developed. The aim of this chapter is to provide an overview of squamous cell carcinoma and adenocarcinoma of the esophagus.
synonymous with squamous cell carcinoma. Adenocarcinoma of the esophagus and the gastroesophageal junction are now more frequent in the United States and represent the fastest-growing solid malignancy. According to the American Cancer Society, the estimated incidence of esophageal cancer in the United States for 2008 was 16,470, with approximately 14,280 cancer-related deaths.11 Worldwide the total number of new cases of esophageal cancer was estimated at 500,000.158 The most important prognostic factor for esophageal cancer remains the stage of disease at the time of diagnosis. Currently, the best strategy for improving survival is early diagnosis. As the molecular biology of esophageal cancer is better understood, novel diagnostic, therapeutic, and prognostic modalities continue to be developed. The aim of this chapter is to provide an overview of squamous cell carcinoma and adenocarcinoma of the esophagus.
Squamous Cell Carcinoma of the Esophagus
Epidemiology
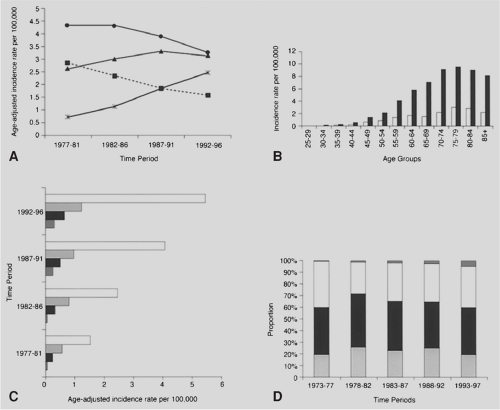
In the United States, the overall annual incidence of squamous cell carcinoma is 2.6 per 100,000 according to Yang and Davis,312 as shown in Figure 158-1 for the years 1977 to 1996. The age-specific rates are extremely low in persons <40 years of age and continue to rise with each decade of life. The incidence of esophageal cancer varies among different racial groups and is four to five times higher among African Americans than Caucasians; it has become the second most common malignancy among African-American men <55 years of age, as noted by Blot and Fraumeni.29 Age-adjusted mortality for squamous cell carcinoma has remained relatively stable among Caucasians, whereas the rate among African Americans has nearly doubled over the same 30-year period. It has been shown that regardless of race, men are affected three to four times as often as women.46,312 It also appears that the overall 5-year survival rate for squamous carcinoma of approximately 5% may be slightly improving.
There appear to be geographic and cultural variations in the incidence of squamous cell carcinoma of the esophagus, suggesting that environmental exposure is causally important.239 Regions with a high incidence are generally located in poorer parts of the world, often associated with nutritional deficiencies. In China, approximately 60% of the world’s cases occur annually, the incidence is clustered into sharply demarcated geographic areas. Areas located in the southern parts of the Taihang Mountains on the borders of Henan, Shansi, and Hopei provinces in China have some of the highest incidence and mortality rates of esophageal SCC in the world.269 In Central Asia, an esophageal cancer belt extending from northern Sinkiang through the former Soviet republics of Kazakhstan, Uzbekistan, and Turkmenistan and including northern Afghanistan and northeastern Iran has been described.60 High rates have also been noted in the Indian subcontinent, and intermediate to high rates exist in the Caribbean and portions of Latin America.290 In the United States, where the disease is relatively uncommon, urban African-American men seem particularly affected, especially in Washington, D.C., and coastal South Carolina.87
Etiology
Nutrition
Worldwide, nutritional deficiencies have been implicated in the pathogenesis of esophageal squamous cell carcinoma, along with a number of other etiologic factors listed in Table 158-1. Low levels of retinol, riboflavin, ascorbic acid, and alpha-tocopherol are prevalent among the people of Linxian, China, where esophageal cancer is endemic has been noted312 Diets low in fruits, particularly citrus, and low vitamin C intake have been repeatedly associated with an increased risk for esophageal
cancer.106 Deficiencies in various mineral elements, such as selenium, zinc, and molybdenum, have also been identified as possible etiologic factors.36,132.These deficiencies are believed to make one more susceptible to the carcinogenic effects of exogenous factors.
cancer.106 Deficiencies in various mineral elements, such as selenium, zinc, and molybdenum, have also been identified as possible etiologic factors.36,132.These deficiencies are believed to make one more susceptible to the carcinogenic effects of exogenous factors.
Table 158-1 Etiologic Factors in Squamous Cell Carcinoma of the Esophagus | |
|---|---|
|
Environmental Carcinogens
Nitrates and nitrites can be converted within the body to carcinogenic N-nitrosamines and are suspected etiologic factors in the development of esophageal cancer. Low molybdenum levels in the soil may cause higher nitrate and nitrite levels in plants because molybdenum functions as a cofactor for the plant enzyme nitrate reductase.36 Citrus fruits and vitamin C, on the other hand, may inhibit endogenous nitrosation and reduce the risk for esophageal squamous cell carcinoma.179 A correlation has also been found between the high prevalence of esophageal cancer in the Gassim region in Saudi Arabia and contamination of water by impurities such as petroleum.10
Alcohol and Tobacco
Numerous studies have linked alcohol and tobacco use with the development of esophageal cancer in Western countries.207,313,315 Ziegler315 found that ethanol is associated with nearly 80% of the neoplasms among esophageal cancer patients, and the relative risk increases with the amount of alcohol consumed. There is also an association between increasing alcohol consumption and poor nutrition. The risks associated with tobacco use appear to increase with the number of cigarettes smoked per day, duration of smoking, and tar content. Opium smoking may also produce potentially carcinogenic substances by pyrolysis and has been implicated in the etiology of esophageal cancer in northern Iran.95 Ex-smokers have a reduced risk, and after 10 years, their risk returns to baseline. A synergistic effect of alcohol consumption and tobacco use has been reported.207 Among well-nourished North American and European men who do not smoke or consume alcohol, esophageal squamous cell carcinoma is virtually nonexistent.60
Achalasia
Achalasia also appears to be a risk factor for the development of esophageal cancer, with a prevalence of about 3% to 6%. They are estimated to have a 30-fold greater likelihood of developing esophageal cancer than the normal population.195 The average duration between symptoms and detection of esophageal carcinoma is 17 years, and esophageal cancer occurs at an earlier age in patients with achalasia when compared with the remainder of the population.40 In patients treated with dilation or esophagomyotomy, esophageal cancer developed at a rate only slightly higher than that for the general population.311 Current clinical guidelines of the American Society for Gastrointestinal Endoscopy12 suggest endoscopic surveillance for untreated patients with achalasia.
Caustic Injury
The incidence of esophageal cancer among patients with a history of caustic ingestion has been estimated to be 1,000-fold greater than that of the general population.126 Squamous cell carcinoma is the most common subtype and typically occurs in the middle third of the esophagus at the level of the tracheal bifurcation.129 Most cases present four to five decades after the initial injury and 10 to 20 years earlier than in the general population.16 Carcinoma should be suspected with any change in the ability to dilate a chronic stricture or in the presence of increasing dysphagia.
Scarring of the esophagus may actually alter the natural history of the esophageal cancer. Because the lumen is less distensible, dysphagia is more likely to present earlier in the course of the disease. In addition, damage to submucosal lymphatics and the presence of dense scar tissue within the esophageal wall may limit lymphatic spread.161
Genetic Factors
The molecular biology of esophageal cancer is an area of active research. Much research has centered around p53, a tumor suppressor gene important in cell cycle control, DNA repair and synthesis, genomic stability, and apoptosis. p53 mutations and allelic losses are common abnormalities in many human neoplasms. Wang and colleagues297 found p53 mutations in the early stages of human esophageal carcinogenesis and postulated that independent somatic mutations in different regions of the esophagus might be key molecular events in multifocal esophageal carcinogenesis. Chaves and coworkers49 found p53 in 57% of esophageal cancer patients, whereas others reported increased p53 levels in all patients with dysplasia.249 Mutant p53 protein expression was also increased in the nonmalignant mucosa of patients after gastrectomy (68%) and in
patients with advanced achalasia (44%), two conditions considered to be risk factors for esophageal squamous cell carcinoma. Mutant p53 overexpression has been shown to correlated with poorer patient survival.285 Mutations of the TP53 gene appear to link chronic inflammation with cancer; mutations such as G:C and A:T transitions at CpG dinucleotides were highly prevalent in SCC in high-risk parts of Asia.159
patients with advanced achalasia (44%), two conditions considered to be risk factors for esophageal squamous cell carcinoma. Mutant p53 overexpression has been shown to correlated with poorer patient survival.285 Mutations of the TP53 gene appear to link chronic inflammation with cancer; mutations such as G:C and A:T transitions at CpG dinucleotides were highly prevalent in SCC in high-risk parts of Asia.159
Tylosis, an autosomal dominant disorder characterized by hyperkeratosis of the palms and soles, appears to be the only well-documented genetic disorder associated with esophageal cancer.256 Affected patients have an about 95% chance of developing cancer during their lifetimes.113 Haunait and Lambert reviewed causes of esophageal cancer and found an association with a locus on chromosome 17p (TOC locus). In addition, they noted that in smokers, gene polymorphisms of the CYP2A6 gene could play a role in the formation of carcinogenic nitrosamines.160
Miscellaneous
Esophageal cancer has also been associated with several other risk factors. Patients with a history of exposure to ionizing radiation, head and neck cancer, Plummer–Vinson syndrome, celiac disease, and thyroid disease have been reported by many to be at increased risk for developing esophageal squamous cell carcinoma.17,19,257,259 Women who receive radiation for breast cancer were found to have an increased risk for esophageal cancer.2
Pathology
Macroscopic Features
Esophageal squamous cell carcinoma is most commonly located in the middle third of the thoracic esophagus and was the site of about 50% of squamous cell lesions.229 This region of the esophagus extends from the level of the carina to the inferior pulmonary veins. Thirty to 40% of lesions arise in the lower third of the esophagus, including the abdominal portion, whereas only 10% to 20% of squamous cell carcinomas occur in the upper esophagus.
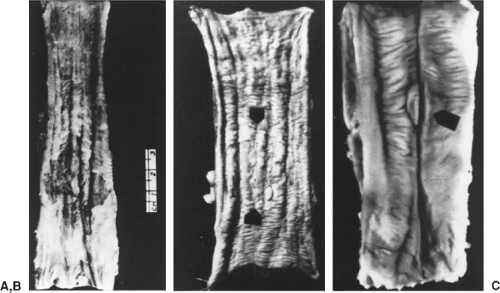
The early stages of esophageal carcinoma are infrequently encountered in North America and Europe and are seen more commonly in China and other endemic areas where routine screening is practiced. The early lesions are generally small but may involve the entire circumference of the mucosa and appear as plaque-like, erosive, or papillary lesions, as shown in Figure 158-2. Early cancers may also be occult with no grossly visible lesions and generally invade only the mucosa and submucosa, although the papillary type may invade the muscular wall of the esophagus.
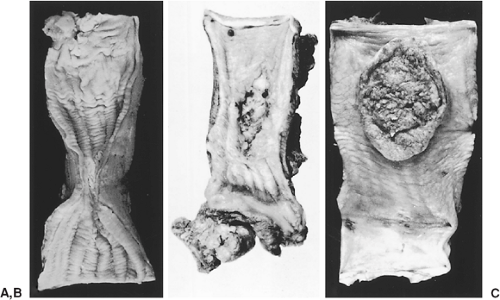
More advanced lesions have been described as fungating, ulcerative, or infiltrative tumors, as demonstrated in Figure 158-3.188 Fungating lesions project into the lumen and appear as filling defects on barium swallow. These lesions may also present as flat plaques. Ulcerated lesions have regular or irregular everted edges with a deep base and associated infiltration of the esophageal wall. Infiltrative tumors are characterized by extensive intramural spread. A desmoplastic response is usually present and can produce a tight esophageal stricture. Occasionally, tumor spread is only superficial, resulting in the superficial spreading type. Other investigators have used a variety of gross classification schemes. Akiyama3 divided the various types into protuberant, ulcerative, superficial, exophytic, and miscellaneous types. Liu and Zhou177 classified squamous cell carcinomas into medullary, fungating, ulcerative, scirrhous, and intraluminal lesions. Descriptions of the different types overlap, but the extent of invasion and the presence or absence of lymph node metastases are the critical features that determine patient prognosis.
Microscopic Features
Early squamous cell carcinomas can be divided into intraepithelial, intramucosal, and submucosal tumors. Intraepithelial lesions are typical carcinoma in situ with an intact basement membrane. In intramucosal carcinomas, the tumor cells penetrate the basement membrane and infiltrate the lamina propria or part of the muscularis mucosa. Once the cells have
penetrated the muscularis mucosa, the lesions are classified as submucosal carcinomas, as depicted in Figure 158-4. In the more advanced stages of squamous cell carcinoma, there is invasion into or through the muscular layers of the esophagus and into the adventitia. The degree of tumor differentiation varies from well to poorly differentiated, with 60% of tumors being moderately differentiated.
penetrated the muscularis mucosa, the lesions are classified as submucosal carcinomas, as depicted in Figure 158-4. In the more advanced stages of squamous cell carcinoma, there is invasion into or through the muscular layers of the esophagus and into the adventitia. The degree of tumor differentiation varies from well to poorly differentiated, with 60% of tumors being moderately differentiated.
Metastases
In the advanced pathologic stages of the disease, direct extension through the wall of the esophagus is common, as are lymphatic metastases. Lymphatic metastases were found in about 60% of patients undergoing esophagectomy.3 In an autopsy series, lymphatic metastasis were seen in 75%.187 Hematogenous spread was also common in autopsy specimens, with an incidence ranging from 50% to 63%.17,187
Intraesophageal Spread
Microscopically, most tumors have spread more extensively than their gross appearance would indicate. Wong309 noted the correlation between the proximal resection margin and the incidence of anastomotic recurrence as shown in Table 158-2. Microscopic spread distal to the gross tumor, for reasons that are unclear, extends for a shorter distance of approximately 4 cm from the tumor.35 Submucosal lymphatic spread occurs often and may result in tumor emboli producing satellite nodules with significant prognostic implications.300
Direct Extension
After penetration of the adventitial layers covering the esophagus, the tumor may invade adjacent structures, including the pleura, trachea, left mainstem bronchus, pericardium, great vessels, thoracic duct, and the anterior ligaments of the vertebral column. In the upper esophagus, the recurrent laryngeal nerves may also be involved, whereas in the lower esophagus, tumors may invade the diaphragm, stomach, and liver. However, it has been noted at postmortem examination that the extent of invasion in one-third of the tumors was restricted to the periesophageal tissues.246
Lymphatic Spread
The direction of flow in the extensive lymphatic network of the esophagus is primarily longitudinal. Using lymphoscintigraphy, Tanabe273 demonstrated that lymph from the upper third of the esophagus drains primarily to the upper mediastinum and
neck, whereas lymph from the lower esophagus flows to the abdomen. However, despite the predominantly unidirectional flow seen normally, cervical, supraclavicular, and abdominal lymphadenopathy are seen in both upper and lower esophageal cancers.
neck, whereas lymph from the lower esophagus flows to the abdomen. However, despite the predominantly unidirectional flow seen normally, cervical, supraclavicular, and abdominal lymphadenopathy are seen in both upper and lower esophageal cancers.
Table 158-2 Proximal Resection Margin and Anastomotic Recurrence | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||
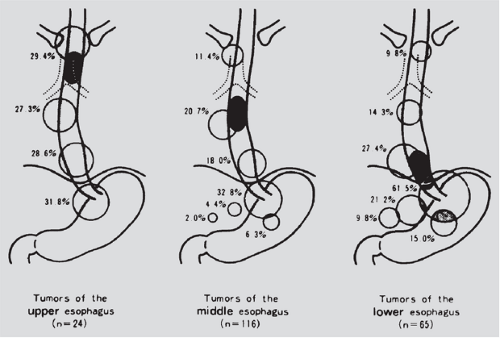
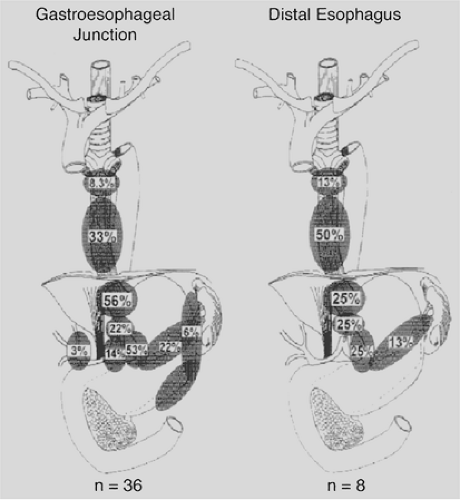
As shown in Table 158-3, the incidence of lymphatic metastases in surgical specimens ranges from 30% to 70% and is related to the depth of invasion of the primary tumor.180 Postlethwait229 found supraclavicular nodal involvement in 6.9% based on a series of autopsy studies. In two smaller surgical series, supraclavicular nodal involvement was seen in about 19%.122,251 Kato and colleagues139 reported that 26% of patients who underwent resection of a carcinoma in the thoracic portion of the esophagus and bilateral cervical lymph node dissection had one or more involved cervical nodes. In contrast, spread below the diaphragm is more common. Akiyama and associates4 reported nodal metastases in the superior epigastrium in 31.8% of patients with tumors located in the cervical esophagus, 32.8% with tumors in the middle third, and 61.5% with tumors in the lower third of the esophagus, as depicted in Figure 158-5.
Distant Metastatic Disease
Visceral metastases may be present in up to 30% of patients at the time of diagnosis and are manifestations of advanced disease. In an autopsy series by Mandard and associates,187 40% of the patients with well-differentiated squamous cell carcinoma of the esophagus had visceral metastases, and 87% of those with undifferentiated squamous cell carcinoma had widespread disease. Metastases were found in the lungs, liver, pleura, bone, kidneys, and adrenal glands, in order of decreasing frequency. The nervous system was involved in 2.7% of specimens, excluding the undifferentiated tumors and a 1% incidence of metastases to the brain has been reported.13
Table 158-3 Degree of Invasion and Lymph Node Status of 504 Resected Specimens of Esophageal Cancer | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||
Esophageal Adenocarcinoma
Esophageal adenocarcinoma is the most rapidly increasing solid malignancy in the United States and is the seventh leading cause of cancer-related deaths.135 There were approximately 12,600 deaths from esophageal carcinoma in 2002. In the mid-1990s, adenocarcinoma overtook squamous cell carcinoma as the most common cancer of the esophagus in America.28,68 Surgical resection remains the only chance for cure, and approximately 90% of those diagnosed ultimately die of their disease. Although there have been significant improvements in surgical technique and perioperative care, the prognosis remains poor, with an overall 5-year survival rate of only 5% to 12%.191 However, new multimodality treatments as well as novel diagnostic and prognostic techniques are being developed, providing hope that survival rates will improve in the future.
Epidemiology
The incidence of esophageal adenocarcinoma has increased progressively since the 1970s. According to the Surveillance, Epidemiology, and End Results (SEER) study, the incidence has increased more than 350% from 0.7 per 100,000 between 1974 and 1976 to 2.5 per 100,000 between 1992 and 1996, as dipicted in Figure 158-1.68,79 The reason for these increases is unclear. Although the use of diagnostic techniques such as endoscopy has increased, it is believed that there has been a true increase in incidence. The median age at diagnosis was 68 years among patients entered into the SEER database after 1988.76 Men are affected six to eight times more often than women, and
Caucasians are affected three to four times as often as African Americans. Incidence rates are also higher in developed countries, and there may be geographic variations. According to SEER data from 1973 to 1998, the incidence in Seattle was twice as high as in Utah, with an 800% increase versus 300%.150 Rates of esophageal adenocarcinoma were also significantly higher among African American males in Connecticut, at 3.1 per 100,000 versus 0.8 per 100,000 nationwide.
Caucasians are affected three to four times as often as African Americans. Incidence rates are also higher in developed countries, and there may be geographic variations. According to SEER data from 1973 to 1998, the incidence in Seattle was twice as high as in Utah, with an 800% increase versus 300%.150 Rates of esophageal adenocarcinoma were also significantly higher among African American males in Connecticut, at 3.1 per 100,000 versus 0.8 per 100,000 nationwide.
Etiology
Barrett’s Metaplasia
In an autopsy study in 1990, Barrett’s metaplasia was found in 0.4% or 376 per 100,000 Caucasians, which is significantly higher than the clinically diagnosed prevalence of only 80 per 100,000.38 This suggests that most cases of Barrett’s esophagus have not been diagnosed. There are about 3 million Americans with Barrett’s metaplasia.39
Barrett’s mucosa is the precursor lesion to esophageal adenocarcinoma, as illustrated in Figure 158-6, and the risk is increased 30 to 125 times the age-matched population with an incidence of one to two cancers per 100 patient-years.37,209 It is more common in males, with a 2:1 ratio and a mean age of diagnosis of 62 years in males and 67 years in females. Esophageal adenocarcinoma develops in about 7% to 20% of patients with Barrett’s metaplasia.109 Although Barrett’s epithelium is seen in 24% to 64% of surgical specimens, its absence in the remaining specimens may be due to overgrowth of the Barrett’s mucosa by tumor or sampling errors.107,253
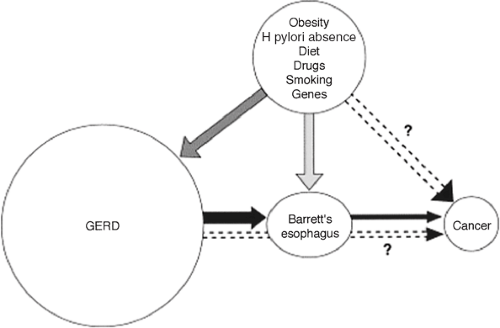 Figure 158-6. Risk factors leading to esophageal adenocarcinoma. (From El-Serag HB. The epidemic of esophageal adenocarcinoma. Gastroenterol Clin North Am 2002;31:425. With permission.) |
On endoscopy, these lesions appear as red, velvety areas. In the past, Barrett’s mucosa has been described as columnar epithelium extending >3 cm above the gastroesophageal junction and was originally believed to be congenital. However, short-segment Barrett’s <3 cm above the gastroesophageal junction has been increasingly recognized as a risk factor for esophageal adenocarcinoma, and 35% of such tumors arise in short-segment Barrett’s.131 Although there are three types of columnar epithelium—including the gastric fundic, gastric junctional, and intestinal type—only the intestinal type is associated with an increased risk of esophageal adenocarcinoma.
Barrett’s esophagus appears to be an acquired condition resulting from chronic inflammation due to gastroesophageal reflux. It has been reported that 18% of the U.S. population experiences heartburn once per week and that 7% have daily symptoms.178,203 At endoscopy for reflux, Barrett’s mucosa was found in 12% to 18% of patients.265,307 A large population study in Sweden revealed an odds ratio for esophageal adenocarcinoma of 7.7 for recurrent reflux and 44 for long-standing reflux.157 There may also be a hereditary component to gastroesophageal reflux, with first-degree relatives 2.2 to 4.8 times as likely to have reflux symptoms.248,279
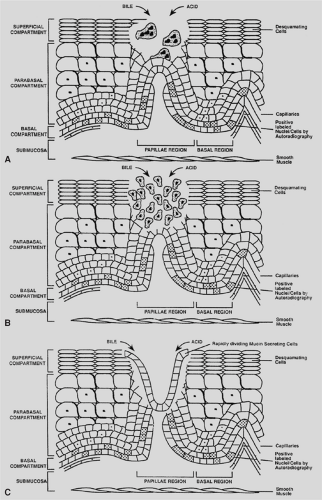
It is now well accepted that reflux of both acid and bile have a major role in the development of Barrett’s esophagus. Although the esophageal mucosa appears to be relatively resistant to acid except at high concentrations, mixed reflux with bile acids, pepsin, trypsin, gastric acid, and lysolecithin appears to be more harmful and has been implicated in mucosal injury, as depicted in Figure 158-7.140 Although primary bile acids are not carcinogenic, it has been noted that secondary bile acids are potential carcinogens, depending on the pH and conjugation status.142 One study showed that 67% of patients with Barrett’s metaplasia have duodenogastric reflux stained with bile.92
Surveillance of Barrett’s Metaplasia
Although there is no direct evidence supporting surveillance programs for Barrett’s mucosa, indirect evidence is available. Esophageal adenocarcinoma is believed to have a 4- to 5-year preclinical phase, indicating that surveillance may be effective.105 Cancers detected during surveillance tend to be less advanced and to have a better prognosis, with a 5-year survival rate of 35% to 45%.230,270 However, these nonrandomized studies must be evaluated with caution, since they may be affected by lead-time bias. It has been noted that the endoscopist’s impression is often incorrect, with a positive predictive value in one study of only 34% as compared with histopathology.74 Others argue that current surveillance measures have had a minimal impact on public health. In a literature review of 752 studies including 1,053 cases, Dulai and associates71 reported that only 4.7% ± 2.9% of patients had a diagnosis of Barrett’s metaplasia preoperatively.
Despite these limitations, surveillance is currently the standard of care. This should start with an initial endoscopy for documentation; if endoscopy shows esophagitis and inflammation, biopsies are not performed. The patient should be treated with acid suppression therapy and repeat endoscopy in 1 to 3 months. On repeat endoscopy, four quadrant biopsies every 2 cm should be done.190 If biopsies show Barrett’s without dysplasia, repeat endoscopy with biopsy is scheduled every 3 years.
If biopsy shows low-grade dysplasia on two different occasions, endoscopy and biopsy are scheduled on an annual basis. However, some would treat such patients with 3 months of acid suppression. If changes persist on biopsies, DeMeester66 suggests an antireflux procedure with continued surveillance every 6 months. If lesions continue to persist, Salo and associates249 recommend consideration for mucosal ablation.
For high-grade dysplasia, options for continued surveillance should be considered in patients who are willing to be involved in an intense surveillance program and be candidates for esophagectomy. For those patients, repeat endoscopy with four quadrant biopsy every 1 cm instead of 2 cm should be scheduled every 3 months.190 Esophagectomy is an option for patients who are good candidates for surgery. Mucosal ablation is another option with long-term results yet to be determined.
Antireflux Therapy for Barrett’s Metaplasia
Although medical and surgical antireflux therapies are often evaluated in terms of symptomatic relief, the goals in the management of Barrett’s mucosa also include prevention, healing, and stabilization of metaplastic lesions. Wetscher and associates303 found that up to 34% of patients developed Barrett’s metaplasia while on long-term acid suppression. Sharma and coworkers258 found a 5.7% incidence of progression to dysplasia and concluded that medical therapy does not prevent the development of dysplasia.
Proponents of antireflux surgery argue that medications do not correct the underlying reflux because acid is not the only injurious agent. Antireflux surgery restores lower esophageal sphincter function, and randomized studies have confirmed superior reflux control.218,264 In a series of 21 patients, Luostarinen and associates186 performed follow-up esophagoscopy 20 years after Nissen fundoplication. Two of the 15 patients with intact repairs had Barrett’s epithelium, whereas 5 of 6 patients with defective wraps showed Barrett’s metaplasia. In a literature review, DeMeester and DeMeester63 found that 13 of 340 patients had complete regression, whereas 256 remained unchanged.
Surgical therapy may also prevent progression of Barrett’s metaplasia to dysplasia and cancer. DeMeester and associates62 described 60 patients who underwent antireflux surgery, 10 of whom were found to have low-grade dysplasia preoperatively.
On postoperative endoscopy, 7 of 10 had reverted to Barrett’s epithelium. McCallum and associates193 described a series of 181 patients. Of the 29 who underwent surgical therapy, 2.4% developed dysplasia. In the medically treated group, 19.7% developed dysplasia. No adenocarcinoma was found in the surgical group as opposed to the medically treated patients. Hofstetter and colleagues112 found no high-grade dysplasia or adenocarcinoma in 410 patient-years of follow-up and concluded that antireflux surgery results in long-lasting relief and prevention of high-grade dysplasia and adenocarcinoma.
On postoperative endoscopy, 7 of 10 had reverted to Barrett’s epithelium. McCallum and associates193 described a series of 181 patients. Of the 29 who underwent surgical therapy, 2.4% developed dysplasia. In the medically treated group, 19.7% developed dysplasia. No adenocarcinoma was found in the surgical group as opposed to the medically treated patients. Hofstetter and colleagues112 found no high-grade dysplasia or adenocarcinoma in 410 patient-years of follow-up and concluded that antireflux surgery results in long-lasting relief and prevention of high-grade dysplasia and adenocarcinoma.
Spechler and associates,266 however, found no significant differences among 247 patients randomized to medical or surgical therapy in a Veteran’s Administration study and concluded that antireflux therapy does not prevent adenocarcinoma.
A recent systematic review of more than 1,600 patients was performed; 700 patients were identified in the surgical group and 996 patients in the medical group.48 Data suggested that antireflux surgery is associated with regression of Barrett’s esophagus and/or dysplasia. However, evidence suggesting that surgery reduces the incidence of adenocarcinoma is largely driven by uncontrolled studies. Objective measures and precise definitions of reflux and regression of Barrett’s epithelium are needed before firm conclusions can be made.
Chemoprevention
Cycloxygenase-2 (COX-2) has been found to be upregulated in Barrett’s metaplasia and continues to increase in the progression to dysplasia and adenocarcinoma.306 Bile acids may activate COX-2 through the protein kinase C pathway.314 These changes may result in decreased cell–cell adhesion, increased angiogenesis, or inhibited apoptosis. In one study, aspirin and specific COX-2 inhibitors were found to suppress growth and induce apoptosis.263 In a large case-control study, Corley and associates58 found that this effect was more prominent for squamous cell carcinoma rather than adenocarcinoma and aspirin rather than NSAIDs. Mehta and associates194 suggested that the effect of NSAIDs might be through a COX-independent pathway. A study from the Netherlands suggests that COX-2 mediated inflammatory changes are important in facilitating a microenvironment that promotes carcinogenesis200. Their data supports the beneficial effect of NSAID-induced COX-2 inhibition for prevention of esophageal adenocarcinoma. More studies are needed to prove this effect and its future use for chemoprevention.
Other Risk Factors
Associations have also been found between obesity and esophageal adenocarcinoma. Esophageal adenocarcinoma was 7.6 times more common in the heaviest quartile in a Swedish study.156 Other risk factors include ectopic gastric mucosa and esophageal diverticula. There may also be associations with iron overload, alcohol use, polysaturated fats, and diets high in red meat.92,298 In a study from the National Cancer Institute, a 2.2 times higher risk for esophageal adenocarcinoma was reported in smokers that persisted for up to 30 years after quitting.92 In addition, there has been an increased risk of esophageal adenocarcinoma in patients on medications that reduce lower esophageal sphincter pressure, such as calcium channel blockers.157
Concerns have also been raised about acid-suppressing medications and continued reflux. In addition, there is concern that eradication of Helicobacter pylori may actually lead to increased esophageal adenocarcinoma. The acid-suppressing effects of Cag A+ strains have been inversely correlated with esophageal adenocarcinoma.50 Erosive esophagitis has been found in 26% of patients who were cleared of H. pylori compared with 13% of those with persistent infections.154 In the United States, increasing obesity and decreasing H. pylori infections could contribute further to the rising incidence of esophageal adenocarcinoma.
Pathogenesis
After the squamous epithelium is injured by reflux, there is an inflammation-stimulated hyperplasia and metaplastic change to a columnar epithelium. One hypothesis is that pluripotent stem cells in the basal layers undergo metaplasia after repeated stimulation from reflux. Other possible origins of columnar cells are the gastric cardia and propagation of columnar cells from the esophageal gland ducts. Replacement by long-segment Barrett’s is believed to occur rapidly and reaches its maximal proximal extent within 3 years.201 With further injury and multiple genetic changes, further progression occurs in a stepwise fashion in a metaplasia–dysplasia–adenocarcinoma sequence.94 A recent study suggest that cells of bone marrow origin could contribute to esophageal regeneration and metaplasia in rats.252
Molecular Basis
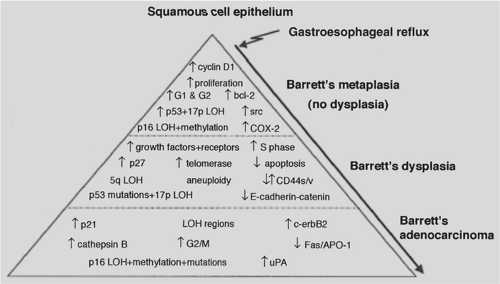
Carcinoma that arises in the setting of Barrett’s metaplasia is thought to develop as part of the metaplasia-dysplasia-carcinoma sequence. It seems that the accumulation of several changes in gene and protein rather than the sequence of events is more essential.147 Some of these changes are depicted in Figure 158-8.305
Various growth factors and receptors are increased in the progression to esophageal adenocarcinoma including epidermal growth factor (EGF), EGF receptor, and fibroblast growth factor (FGF).33,262 EGFR gene amplification is correlated with lymph node invasion, and its overexpression with decreased postoperative survival in patients with locally advanced cancer treated with preoperative chemo and radiotherapy.96 C-erb B2 (Her2/neu) protein overexpression was found in 10% to 70% of esophageal adenocarcinoma; however, it was not demonstrated in metaplastic or low-grade dysplastic Barrett’s suggesting that it is a late event.108,294 Furthermore, C-erb B2 amplification independently predicted poor outcome and short survival in patients with Barrett’s-associated adenocarcinoma.32 C-myc upregulation was found in 50% of metaplasia and 90% of adenocarcinoma with a role for acidified bile as an agent responsible for inducing this oncogene.281
Tumor suppressors—including p16, Rb, VHL, CDKN2, and DCC—are decreased in adenocarcinomas.21,57,131 APC, another tumor suppressor, accumulates in the nucleus in its mutated form.24 Changes have also been seen in cell–cell adhesion, facilitating tumor invasion; decreased expression of E-cadherin and β-catenin have been correlated with a poor prognosis.20
Inhibition of apoptosis is also seen in dysplastic cells as well as an increase in apoptotic rate with increasing histological severity. Decreased expression of Fas receptor at the cell surface in dysplasia and adenocarcinoma, leads to a decrease in apoptosis.115 Bcl-2, an antiapoptotic protein, was found to
be overexpressed in 72% of Barrett’s metaplasia and 100% of low-grade dysplasia, although only 20% to 40% of high-grade dysplasia or adenocarcinoma showed overexpression.137 Bcl-2 may be important early in the dysplasia–carcinoma sequence in allowing dysplastic cells to proliferate. The p53 gene is one of the most commonly mutated genes in cancer. Accumulation of mutated p53 increases with progression to dysplasia and adenocarcinoma, and loss of heterozygosity is found in more than 50% of adenocarcinomas.9,98 Koppert and associates concluded that, P53 gene alterations are early and frequent events in esophageal adenocarcinoma and it is associated with the malignant transformation of Barrett’s esophagus. It could be of value in the prevention as well as treatment planning in future studies
be overexpressed in 72% of Barrett’s metaplasia and 100% of low-grade dysplasia, although only 20% to 40% of high-grade dysplasia or adenocarcinoma showed overexpression.137 Bcl-2 may be important early in the dysplasia–carcinoma sequence in allowing dysplastic cells to proliferate. The p53 gene is one of the most commonly mutated genes in cancer. Accumulation of mutated p53 increases with progression to dysplasia and adenocarcinoma, and loss of heterozygosity is found in more than 50% of adenocarcinomas.9,98 Koppert and associates concluded that, P53 gene alterations are early and frequent events in esophageal adenocarcinoma and it is associated with the malignant transformation of Barrett’s esophagus. It could be of value in the prevention as well as treatment planning in future studies
Other chromosomal changes seen in esophageal adenocarcinoma include a number of genomic amplifications, deletions, and microsatellite instability.5,172,173 It has been suggested that nuclear DNA ploidy analysis on flow cytometry might provide a more objective and reliable biomarker associated with progression from metaplasia to carcinoma than the histological diagnosis of high-grade dysplasia.80,238 Loss of chromosome Y is found in 31% to 93% of tumors.305 In addition, repression of gene expression through promoter hypermethylation is frequently seen. Hypermethylation of the APC promoter has been found in 94% of esophageal adenocarcinomas and found an association with advanced stage and reduced survival.141
Using clonal ordering of neoplastic lineages, numerous intermediate clones were identified, suggesting multiple genetic routes to cancer from Barrett’s metaplasia.22 In one pathway, diploid metaplastic cells developed loss of heterozygosity at 17p and 9p with mutations in p53 and p16. Cell populations then arose, with an elevated 4N fraction. These cells developed aneuploid populations that became cancer cells with additional loss of heterozygosity. Studies of the genetic changes involved in the pathogenesis of esophageal adenocarcinoma not only should improve our understanding of this cancer but also may provide novel methods of diagnosis, staging, and treatment.
Pathology
Barrett’s Esophagus
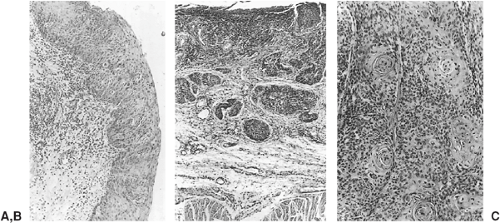
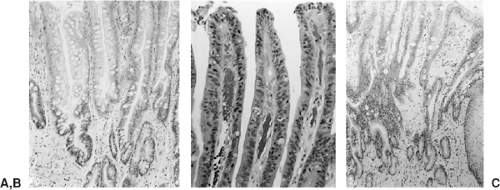
On endoscopy, these lesions appear as red, velvety areas between smooth, pale esophageal squamous mucosa, as shown in Figure 158-9A and B. Microscopically, columnar epithelium is seen with mucosal glands that often contain intestinal goblet cells, as shown in Figure 158-10A. Barrett’s metaplasia is the precursor to esophageal adenocarcinoma, and high-grade dysplasia seen on histopathology remains the best predictor of progression to adenocarcinoma.238 Thee system for dysplasia classification in inflammatory bowel disease was used by Reid and associates236 for Barrett’s esophagus and related dysplasia. Dysplasia is defined as cytologic and structural abnormalities that are distinguishable from reactive changes and extend to the luminal surface, as shown in Figure 158-10B. Lesions are classified as low- or high-grade dysplasia and are distinguished by the nuclear orientation along the base of the epithelium as well as other characteristics, as illustrated in Table 158-4. In fact, lesions are often a mosaic of low- and high-grade dysplasia adjacent to nondysplastic mucosa.144 It is important to remember there can be inter- and intra-observer variability.
Esophageal Adenocarcinoma
Esophageal adenocarcinomas usually arise in the distal esophagus. Based on SEER data, 79.3% of adenocarcinomas arise from the lower esophagus, 17.9% from the middle, and 2.8% from the upper third.312 Lesions are initially flat or raised patches of mucosa that may become infiltrative or deeply ulcerated lesions. Nodular masses up to 5 cm in diameter can also be seen. Microscopically, most tumors are composed of mucin-producing intestinal-type glands, as shown in Figure 158-10C. Diffusely infiltrating gastric-type signet-ring cells can be seen but are less common.
Metastases
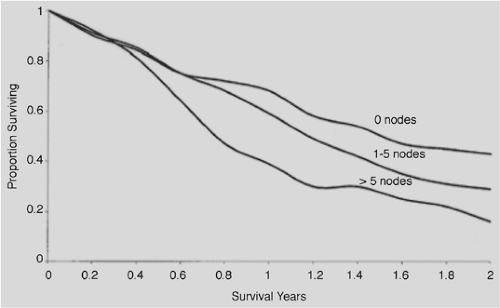
The number of esophageal adenocarcinomas detected at an early stage is small. According to SEER data,191 carcinoma in situ was found in 2.3% of patients between 1993 and 1997. Even when tumors are confined by the muscularis mucosa, 8% to 30% have nodal disease and 30% to 58% have lymph node metastases when the submucosa is involved.103 Of 295 patients, Rice and associates242 found nodal disease in 10% of patients with T1 lesions, 46% of those with T2, and 83% of those with T3. The sites of lymph node metastases are depicted in Figure 158-11. Lymph node status is a major prognostic factor and influences survival, as shown in Figure 158-12.
Lymphatic channels begin in the mucosa and drain into the submucosa, forming long collecting channels. Numerous valves direct the lymphatic flow longitudinally. Although in normal conditions lymph above the carina flows into the thoracic duct or subclavian lymphatic trunks and lymph below the carina drains into the cisterna chyli in the abdomen, reversal of flow can occur when nodes are blocked by tumor. Because of this extensive lymphatic network, a large number of patients have unresectable disease at the time of diagnosis. The most common sites of distant metastases include the liver (35%), lungs (20%), bone (9%), brain or adrenal glands (2%), and pericardium, pancreas, spleen, or stomach (1%), as shown in Table 158-5.233
Adenocarcinoma of the Gastroesophageal Junction
Inconsistent classification schemes and definitions have caused confusion in comparing adenocarcinoma of the esophagus and the gastroesophageal junction. Some studies suggest that risk factors for intestinal metaplasia in the cardia may be different from those involved in Barrett’s mucosa and failed to find a strong association with reflux.77,201 There are also epidemiologic differences. Although the incidence of adenocarcinoma of the cardia has increased, with an incidence of 3.3 per 100,000 from 1987 to 1991, it has increased to a lesser extent and has remained stable according to SEER data between 1987 and 1996.28,30,79 The ethnic and gender differences are also smaller. Patients with intestinal metaplasia of the cardia are more likely to be non-Caucasians and to have gastritis.77 Some studies have found strong associations between H. pylori infection, gastritis, and the presence of intestinal metaplasia in the distal stomach.77,201 Other found common risk factors, a poor prognosis with 5-year
survival rates from 22% to 38%, and increasing incidence rates similar to esophageal adenocarcinoma.30,224
survival rates from 22% to 38%, and increasing incidence rates similar to esophageal adenocarcinoma.30,224
Table 158-4 Grading Dysplasia in Barrett’s Esophagus | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||
Diagnostic Evaluation
Presentation
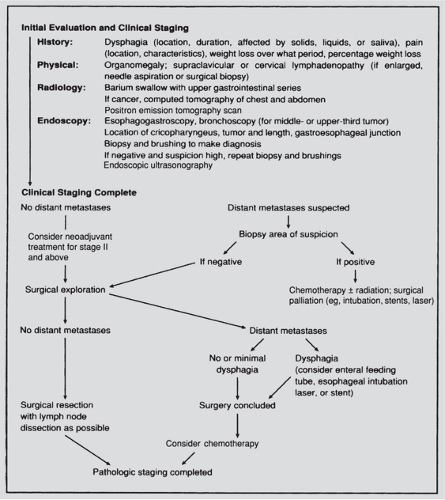
The initial evaluation of a patient with a suspected esophageal carcinoma is depicted in Figure 158-13. The symptoms of esophageal cancer are usually nonspecific and similar regardless of the histologic subtype. Dysphagia is the most common initial symptom. Typically, difficulty swallowing is noted with solid foods, which may progress to include semisolids and eventually liquids over a period of weeks to months. Odynophagia is the next most common symptom and may be caused by an ulcerated lesion or invasion of surrounding mediastinal structures. Constant pain in the midback or midchest also suggests mediastinal invasion. Regurgitation of food immediately after swallowing may occur as the growing neoplasm narrows the esophageal lumen. The constitutional symptoms of anorexia and weight loss are often present by the time the patient seeks medical attention. Hoarseness may also occur with proximal tumors and indicates involvement of the recurrent laryngeal nerves.
Table 158-5 Occurrence of Metastases in Patients with Esophageal Cancer | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||
Patients with esophageal adenocarcinoma often have a history of reflux symptoms, although clinical features do not distinguish patients with and without Barrett’s mucosa, which is frequently asymptomatic. Patients often present at an advanced stage unless the diagnosis is made fortuitously on endoscopy performed for other reasons. The signs and symptoms of advanced esophageal carcinoma are shown in Table 158-6. In a series of
115 patients, van Sandick and associates289 reported dysphagia in 67% of patients, an 11% incidence of retrosternal or epigastric pain, and a 5% incidence of hematemesis or melena. Seven percent of cases were asymptomatic and discovered during surveillance, and 47% of patients experienced more than a 5% weight loss.
115 patients, van Sandick and associates289 reported dysphagia in 67% of patients, an 11% incidence of retrosternal or epigastric pain, and a 5% incidence of hematemesis or melena. Seven percent of cases were asymptomatic and discovered during surveillance, and 47% of patients experienced more than a 5% weight loss.
Table 158-6 Signs and Symptoms Produced by Advanced Esophageal Carcinoma | ||
|---|---|---|
|
Physical examination may reveal entirely normal findings and generally does not aid in the diagnosis. Temporal wasting, weight loss, and dehydration can be seen. It is not clear whether this is simply malnutrition resulting from an obstructed esophageal lumen or whether the tumor is secreting factors that promote cachexia. The ability of patients to gain weight after successful palliation of dysphagia suggests the former. It is important to look for physical findings that may alter the therapeutic approach, including supraclavicular or cervical adenopathy, a discrete abdominal mass, or fullness that may indicate involvement of the celiac nodes or metastatic disease to the liver.
Laboratory examinations may reveal anemia from chronic blood loss, hypoproteinemia from malnutrition, and hypercalcemia and abnormal liver function tests from distant metastases. Hypercalcemia is more common than previously appreciated and was seen in about 15% of patients with esophageal squamous cell carcinoma.22,47,268
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree