Bronchoscopy in the Diagnosis and Evaluation of Lung Cancer
Thomas G. Sutedja
Thomas Santo
Michael Zervos
Felix J.F. Herth
Rigid optics to examine the central airways were designed by Gustav Killian in Heidelberg more than a century ago and were revolutionized with the creation of the fiberoptic bronchoscope by Shigeto Ikeda. 1 Recent and continuing advancements in catheter-based tissue imaging and therapeutics have significantly increased the role of bronchoscopy in the management of lung cancer patients because of its minimal invasive nature. 2,3,4,5,6 Bronchoscopy provides us with less morbid and tailored strategies for each individual at risk with regard to extended diagnostics and therapeutics by the application of many techniques currently available. 3,4,5,6,7 Thus, bronchoscopic activities also encompass early detection, staging, and treatment for at-risk individuals with various pulmonary pathologies, and its role for the management of lung cancer has become an integral part in the thoracic oncology discipline as well. 3,4,7
From the perspectives of interventional pulmonology, target lesions are either centrally located inside or adjacent to the major airways or located in the lung parenchyma beyond the segmental bronchi, in which current fiberoptic bronchoscopes with an average diameter of ≈6 mm have limited access. However, the standard use of fluoroscopy and current advancement of computerized digital four-dimensional (4D) imaging computed tomography (CT), magnetic resonance imaging (MRI), ultrasound, and tissue spectral analysis can be further exploited to refine bronchoscopic applications in studying dynamic disease processes in pulmonary parenchyma beyond our direct vision and toward visualization of subcellular processes. 8,9,10,11,12
The aim of this chapter is to describe current and future perspectives of various bronchoscopic techniques that are used in the management of lung cancer. Recent advancements in nodal staging and therapeutic strategy for early detected lesion will be more extensively discussed.
THE CONCEPT OF BRONCHOSCOPIC DIAGNOSIS, STAGING, AND TREATMENT
For most patients with clinically overt lung cancer based on current World Health Organization (WHO) histology classification, the squamous and small cell types are primarily located in the central airways, whereas adeno and large cell neuroendocrine types are mostly located in the lung parenchyma distal to the segmental bronchi. As the majority of patients are still diagnosed at advanced stage, central bulky tumor and nodal disease involvement adjacent to the central airways can be (re-)staged by acquiring specimens for tissue diagnosis with relative ease. 3,6
Bronchoscopic sampling of specimens for histological evaluation in the central airways can be performed under direct vision, and the use of coagulative techniques (e.g., lasers, electrocautery, and argon plasma) and cryotherapy can better control bleeding that may occur. 3 Extended use of various debulking techniques for obtaining immediate relief in patients with imminent suffocation will not be dealt here (see Chapter 61).
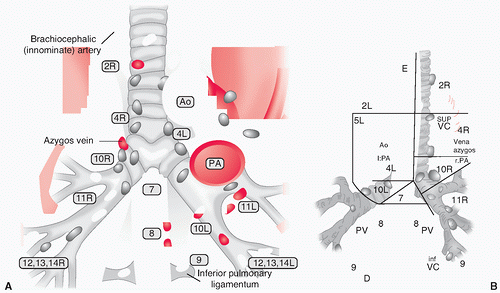
Adjacent to the central airways are the mediastinal lymph nodes (MLN) (Fig. 28.1), which can be staged using transbronchial (and transesophageal) needle aspiration as alternatives for conventional staging (e.g., mediastinoscopy and videoassisted thoracoscopic surgery [VATS]). 6,13,14 These aspiration techniques are a major improvement for reducing morbidity in those with advanced cancers, with improved accuracy and safety caused by the advent of esophageal and endobronchial ultrasound (EUS and EBUS), allowing real-time puncturing of the nodes. 13,14 It is important to know that mediastinal nodes move during respiration, such that in dealing with especially small unforeseen 18F-fluorodeoxyglucose positron emission tomography (FDG-PET)- positive nodes, one cannot presume the locations to be static during aspiration. 15,16 It is therefore quite obvious that with expertise and real-time puncturing of the nodes, diagnosis can be obtained in >90% accuracy. 13,14 These endoscopic alternatives are straightforward and least morbid in staging procedures, despite the great potential of noninvasive imaging techniques such as PET/CT. Tissue will remain the issue for still a considerable period of time as FDGPET avidity is showing metabolic function not exclusively for malignancies alone. 17,18
Currently, for lesions in the lung parenchyma, tissue sampling under fluoroscopy, CT, or ultrasound guided may prevent the need for more invasive surgical diagnostics. 12,19,20 However, 30% of pulmonary parenchymal lesions do not have proximity to the smaller airways. Therefore, transthoracic approaches may still be required with the inherent risk for causing a pneumothorax— a potential complication often overrated as an interventional specialist should anticipate any procedure-related complication.
The issue of huge numbers of submillimeter parenchymal lesions detected in current CT screening programs cannot be easily addressed with either fluoroscopic or endobronchial techniques. Targeting all these lesions will be a colossal task. The entire strategy regarding CT screening requires thorough understanding of all screening controversies (see Chapter 16). 21,22,23,24,25
4D navigational techniques based on CT data seem promising. Nevertheless, the requirement for tissue biopsy should be put in the proper perspective of the CT screening controversies. 25,26,27,28 The issues of potential overdiagnosis, relatively high number of only bronchioloalveolar cell carcinoma (BAC) lesions found, the lack of any proof that stage shift has been accomplished, together with potential difficulty for proper histological classification if only based on tiny pieces of tissue specimens collected, are among the few aspects to consider. 22,25,26,29,30 The poor negative predictive value (NPV) of CT-detected subcentimeter lesions in lung cancer screening study may encourage bronchoscopists to accomplish tissue diagnosis to exclude malignancy, but is practically unrealistic because of the sheer numbers of these lesions that are found, of which most will be nonmalignant. 22,23,24,25 Consideration of theoretical and practical issues should prevent tunnel vision for interventional pulmonologist in dealing with CT screen-detected nodules. 25
When there is a strong suspicion for lung malignancy, PET/CT may soon be expected to become the standard initial staging procedure. 26,27,28 Improved spatial resolution of current PET/CT machines can greatly assist the bronchoscopist for optimal selection of techniques, for example, in using ultrathin bronchoscope, steerable catheters (e.g., virtual bronchoscopic navigation). 19,20,31,32,33 This may ease targeting lesions beyond bronchoscopic reach, thus distal to the segmental bronchi deep in the lung parenchyma, also for first-station nodal disease.
The epidemiological shift of lung cancer cell type to ≈40% adenocarcinoma makes it mandatory for bronchoscopists to be proficient in understanding the potential and limitations of 4D noninvasive spatial data for targeting these lesions. 26,27 Small parenchymal lesions are difficult moving targets because of respiratory cycles and may require adjuncts such as using realtime ultrasound sensor probes. 12,15,16,20 Great promise about the possibility of 4D navigational assistance may improve our ability herein, similar to recent achievements in stereotactic body radiation therapy (see Chapter 43). 34
For central airway lesions within bronchoscopic reach, minute early preneoplastic lesions at the clonal level located
in the bronchial mucosa of the central airways are difficult to detect. 8 Preneoplastic lesions are aberrant clonal cell groups of several hundred cells with an average thickness of five cells only. The role of autofluorescence bronchoscopy (AF) herein has been established. 4 Tumor infiltration beyond the bronchial wall can be visualized accurately using thin cuts high-resolution CT (HRCT), 5 EBUS, 35 with optical coherence tomography (OCT) as a promising tool (see Chapter 19). 36
in the bronchial mucosa of the central airways are difficult to detect. 8 Preneoplastic lesions are aberrant clonal cell groups of several hundred cells with an average thickness of five cells only. The role of autofluorescence bronchoscopy (AF) herein has been established. 4 Tumor infiltration beyond the bronchial wall can be visualized accurately using thin cuts high-resolution CT (HRCT), 5 EBUS, 35 with optical coherence tomography (OCT) as a promising tool (see Chapter 19). 36
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree