Bronchoplastic Procedures
Anna Maria Ciccone
Federico Venuta
Camilla Vanni
Erino A. Rendina
HISTORY
The first sleeve lobectomy was performed by Price-Thomas in 1947 for an endobronchial adenoma. Five years later, Allison reported a sleeve lobectomy for carcinoma. It was Paulson and Shaw, however, who popularized sleeve resection with their 1955 paper, entitled “Bronchial anastomosis and bronchoplastic procedures in the interest of preservation of lung tissue.” The use of their techniques allows the modern thoracic surgeon to perform parenchymal-sparing procedures, even in association with reconstruction of the PA (described for the first time by Allison and Thomas in the 1950s), on patients who would not tolerate a pneumonectomy. In the case of malignancy, sleeve resections may be performed without compromise of oncologic principles.
PRINCIPLES AND JUSTIFICATION
Pneumonectomy is associated with an increased morbidity and mortality when compared with lobectomy and sleeve lobectomy. Thus, in our practice, we make every effort to avoid pneumonectomy. This includes complex bronchoplastic and bronchovascular reconstructions when required. The justification of this approach is simply that by avoiding pneumonectomy we avoid its attendant risks while providing an equivalent cancer operation. In addition, lung-sparing procedures allow us to offer curative operations to patients with poor pulmonary function who would not otherwise tolerate the removal of an entire lung.
PREOPERATIVE ASSESSMENT AND PREPARATION
Our preoperative evaluation includes a complete history and physical examination. Special attention is focused on previous thoracic procedures and chest irradiation. The use of high-dose steroids or systemic illnesses that might interfere with bronchial anastomotic healing is noted. All patients have a chest X-ray, a chest computed axial tomography (CAT) scan, and pulmonary function testing with diffusion capacity. Patients with a diagnosis or suspicion of malignancy also have an extentof-disease workup, which includes a bone scan and magnetic resonance imaging (MRI) of the brain when indicated.
We perform mediastinoscopy selectively in patients with malignant disease. Patients who have mediastinal adenopathy of >1.0 cm on CAT scan undergo mediastinoscopy before thoracotomy. If the mediastinoscopy is negative, we proceed with the thoracotomy. If the mediastinoscopy reveals ipsilateral N2 disease, patients are referred for preoperative chemo- or chemoradiation therapy and return later for resection. Those patients with contralateral N3 disease are referred for chemoradiation therapy and are not offered surgical resection.
ANESTHESIA
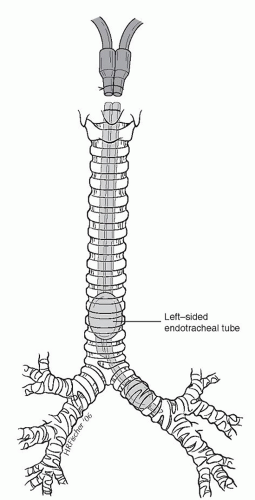
After induction of general anesthesia, all patients undergoing sleeve resection require bronchoscopy by the operating surgeon. This can be done with either a rigid or a flexible bronchoscope. Bronchoscopy allows visualization of the lesion and planning of the resection. After bronchoscopy, it is important for the surgeon to have a complete discussion with the anesthesiologist regarding the operative plan. If a right-sided sleeve resection is contemplated, a left endobronchial double-lumen tube should be placed (Fig. 7.1). If a left-sided sleeve resection is contemplated, a right endobronchial tube is placed. For sleeve pneumonectomy or a carinal sleeve resection, a sterile anesthesia circuit is required to allow direct ventilation from the surgical field.
OPERATIONS
We have performed sleeve resections through standard posterolateral incisions, serratus-sparing posterolateral incisions, and lateral incision, all of which are satisfactory for exposure and dissection.
After entry into the chest, complete exploration is carried out to rule out metastatic disease to either the pleura or lung parenchyma and to assess resectability. On both the right and left side, we begin our dissection in the anterior hilum and completely dissect out the main pulmonary artery (PA). Special care must be taken on the left side to avoid damage to the short left main PA and specifically the apical segmental arterial branch. If there is bulky disease or any difficulty is encountered with dissection, we do not hesitate to open the pericardium on either side to obtain proximal control. Next, we encircle the main PA with an umbilical tape to assure proximal control. The remaining steps are specific to the sleeve resection being performed and each will be described independently in what follows.
Right-Sided Resections
Right Upper Lobectomy Sleeve Resection: The Prototype Bronchoplastic Procedure
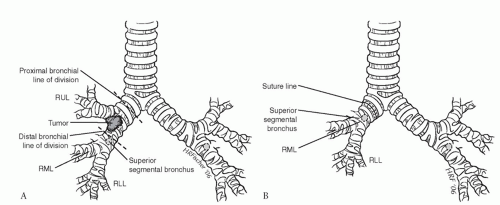
After proximal arterial control has been obtained, we continue our dissection superiorly and enter the plane of the right upper lobe bronchus (Fig. 7.2). The lung is retracted anteriorly, and we continue our dissection in the bifurcation between the right upper lobe bronchus and the bronchus intermedius. A “crotch” lymph node is a consistent finding in this location. This node is elevated away from the bifurcation to reveal the PA branch to the superior segment of the right lower lobe. Once this branch is identified, the posterior portion of the fissure is completed with a linear stapler. This approach avoids extensive parenchymal dissection in the fissure. The bronchus intermedius is encircled just distal to the right upper lobe take-off, and an umbilical tape is placed to aid in dividing the airway at the appropriate time. Up to this point, we have not made
any irreversible maneuvers. A complete inspection is carried out to ensure that all diseases including nodal disease can be removed. Once complete resectability is confirmed, we begin by ligating and dividing the PA branches to the right upper lobe. Similarly, the venous drainage is divided with a vascular stapler taking care to preserve the middle lobe venous drainage. The minor fissure is completed with a linear stapler. The main stem bronchus is encircled at its origin, and an umbilical tape is placed. Before committing to the sleeve resection, it is often productive to divide the right upper lobe bronchus at its origin to see whether the tumor can be cleared with a negative bronchial resection margin. This is especially important with carcinoid tumors, in which the endobronchial component may be attached only at one point and the lesion simply pulled out leaving a clear bronchial margin. Once the bronchus has been opened, the decision to proceed with sleeve resection may be made based on the findings either grossly or microscopically.
any irreversible maneuvers. A complete inspection is carried out to ensure that all diseases including nodal disease can be removed. Once complete resectability is confirmed, we begin by ligating and dividing the PA branches to the right upper lobe. Similarly, the venous drainage is divided with a vascular stapler taking care to preserve the middle lobe venous drainage. The minor fissure is completed with a linear stapler. The main stem bronchus is encircled at its origin, and an umbilical tape is placed. Before committing to the sleeve resection, it is often productive to divide the right upper lobe bronchus at its origin to see whether the tumor can be cleared with a negative bronchial resection margin. This is especially important with carcinoid tumors, in which the endobronchial component may be attached only at one point and the lesion simply pulled out leaving a clear bronchial margin. Once the bronchus has been opened, the decision to proceed with sleeve resection may be made based on the findings either grossly or microscopically.
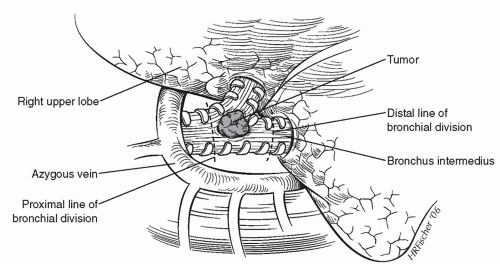
To begin the sleeve resection, we divide the main stem bronchus with a No. 15 blade just proximal to the right upper lobe take-off. Similarly, the bronchus intermedius is divided just distal to the right upper lobe take-off (Fig. 7.3). For upper lobe sleeve resections, these cuts must be perpendicular to the long axis of the airway and placed between cartilaginous rings so as to result in a clean cut. An angled division of the bronchus is to be avoided. The proximal and distal airway margins are cut from the specimen and the true margin inked by the surgeon. We personally take the margins to the pathologist so that proper orientation can be demonstrated prior to frozen-section examination. Once we have documentation that the resection margins are free of tumor, the reconstruction is begun. Microscopic tumor present at a bronchial margin requires additional resection of the involved area or possibly pneumonectomy.
We perform the bronchial sleeve anastomosis in an interrupted manner. The key to a successful bronchial sleeve anastomosis is a pneumostatic wellapproximated, tension-free repair that accounts for any size discrepancy between ends by precise suture placement. We do not take a “tuck” in the proximal airway to make up for a size discrepancy. Rather, we make up the size discrepancy along the entire circumference of the anastomosis by precise suture placement.
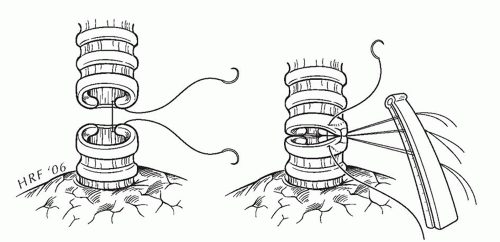
When performing an interrupted anastomosis, we use 4-0 oiled, absorbable, braided suture. The first suture is placed in an “outside-to-in” fashion at the junction of the cartilaginous and membranous bronchi. The suture is not tied but is secured to a suture guide. Additional sutures are placed at 2-mm intervals to complete the first half of the cartilaginous anastomosis (Fig. 7.4). Once the midpoint of the cartilaginous bronchus is reached, we begin tying the sutures starting at the corner. The surgeon “crosses” the next suture in the series to relieve tension while the assistant ties. The cartilaginous anastomosis is completed from the midpoint down to the opposite corner in a similar manner. The lung is retracted anteriorly to reveal the membranous portion of the bronchus. The membranous portion of the anastomosis is completed with interrupted sutures. The chest is filled with saline, and the anastomosis is tested to 20-cm water inflation pressure. Needle hole air leaks are ignored; however, air leaks between the cut edges of the bronchus, if small, are reinforced with simple interrupted sutures. A large area of leaking may require the entire anastomosis to be redone.
We wrap all anastomoses with intercostal muscle (Fig. 7.5), but occasionally we use an omental or pericardial fat
flap. Although ossification of the intercostal muscle flap can occur, it does not necessarily cause problems; in fact, the bronchus has very limited intrinsic motility and a stable caliber. If the intercostal muscle flap is loosely applied around the bronchus, even if some retraction occurs, there is no reason why the hardening caused by ossification should produce stenosis. When the sleeve resection is planned preoperatively, the intercostal pedicle flap may be prepared before the insertion of the rib retractor to avoid crushing the intercostal vascular bundle, and is prepared at full thickness, encompassing a wide pleural flap. The flap is slid backward around the bronchial anastomosis, between it and the PA. The flap is then turned until its pleural side is in contact with the bronchial anastomosis, and the pleura is secured to the bronchus by interrupted sutures.
flap. Although ossification of the intercostal muscle flap can occur, it does not necessarily cause problems; in fact, the bronchus has very limited intrinsic motility and a stable caliber. If the intercostal muscle flap is loosely applied around the bronchus, even if some retraction occurs, there is no reason why the hardening caused by ossification should produce stenosis. When the sleeve resection is planned preoperatively, the intercostal pedicle flap may be prepared before the insertion of the rib retractor to avoid crushing the intercostal vascular bundle, and is prepared at full thickness, encompassing a wide pleural flap. The flap is slid backward around the bronchial anastomosis, between it and the PA. The flap is then turned until its pleural side is in contact with the bronchial anastomosis, and the pleura is secured to the bronchus by interrupted sutures.
Middle Lobe Sleeve Resection
The middle lobe sleeve resection is an infrequently performed resection. After proximal arterial control, the middle lobe vein is identified, isolated, and divided (Fig. 7.6). The bronchus to the middle lobe lies immediately posterior to the middle lobe vein. The bronchus is followed back to its origin. A right-angled clamp is placed around the bronchus intermedius, and it is divided at a location proximal to the middle lobe orifice. The division is slightly angled. The distal division is also angled to preserve the orifice to the superior segment of the lower lobe. The PA lies directly posterior and slightly superior to the bronchus, and care must be taken to avoid injury when dividing the bronchus. After division of the airway, the middle lobe arterial branch is easily visualized. The branch is ligated and divided. Next, the minor fissure and anterior portion of the major fissure are completed with firings of a linear stapler.
After confirmation of negative margins, the airway anastomosis is performed in an interrupted manner as described previously for the right upper lobe sleeve resection. In performing a middle lobe sleeve resection, special consideration must be given to the superior segmental orifice of the lower lobe. One must avoid narrowing the orifice to the superior segmental bronchus when creating an anastomosis. An intercostal muscle flap is used to wrap the anastomosis to separate it from the PA.
Bilobectomy Sleeve Resection
Bilobectomy sleeve resection is performed for an endobronchial lesion in the bronchus intermedius that extends proximally toward the upper lobe orifice (Fig. 7.7). The basic principles of proximal arterial control, microscopic negative margins, and a precise tension-free anastomosis apply. Here the right main stem bronchus is divided just proximal to the right upper lobe take-off and the right upper lobe bronchus is divided at its origin. The right upper lobe bronchus is then anastomosed to the main stem bronchus after removal of the middle and lower lobes, the so-called “Y” sleeve. Due to the reorientation of the upper lobe bronchus after removal of the middle and lower lobes, special care must be taken to avoid torsion of the bronchus at the level of the anastomosis.
Left-Sided Resections
Left Upper Lobe Sleeve Resection
Proximal arterial control is obtained with care to avoid injury to the short apical-posterior segmental branch of the left PA. We continue our dissection along the plane of the artery and identify the superior segmental branch to the lower lobe (Fig. 7.8). At this point, we complete the posterior fissure with a linear stapler. The




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree