Chapter 37 Branched and Fenestrated Grafts for Endovascular Thoracoabdominal Aneurysm Repair
Thoracoabdominal aortic aneurysms (TAA) can extend from the origin of the left subclavian artery to the aortic bifurcation and are categorized according to the Crawford classification.1 Since Etheredge described the first open thoracoabdominal repair,2 perioperative techniques have evolved to limit lower extremity, visceral, and spinal cord ischemia (SCI). Despite the advances, the perioperative mortality risk ranges from 2% to 19%, with the highest risk being derived from statewide Medicare analyses.3–7 The risk of SCI may be as high as 11%, while worsening renal function is seen in up to a quarter of treated patients.5,7–9 On the other end, the risk of rupture of untreated large TAA is considerable and is associated with a dismal prognosis when a patient exhibits ruptures. Even in elective conditions, the magnitude of the open surgical procedure relegates otherwise healthy patients to a considerable risk of complications, while patients with significant comorbidities are generally precluded from repair. Consequently, alternative techniques have been developed to address this complex surgical problem. Hybrid repairs involve the surgical creation of extraanatomic bypasses to the visceral and renal branches based off the distal aorta or iliac artery.10,11 The endovascular portion involves placement of thoracic or bifurcated abdominal graft excluding the involved segment. For less extensive aneurysms that simply abut the visceral or renal arteries, the “snorkel” technique has been described.12,13 This technique involves placement of stents into the target visceral or renal vessel from above that lay parallel to the aortic component, allowing for antegrade flow into the branch vessel and the aortic graft. However, an endovascular seal must be created above the aneurysm, in the region of the snorkel stents or within the neck immediately below the branches. This is not possible in some circumstances, and the durability of such repairs remains in question. Perhaps the simplest conceptual method of endovascular repair of thoracoabdominal aneurysms is that involving fenestrated and branched devices, as they most closely resemble the principles of open surgical repair (SR). The aorta is basically relined, and whenever there is a critical branch, it is incorporated into that repair. Many improvements to the original designs14–17 have been implemented, and the technology is used commercially in Europe, Australia, and areas of Asia. However, dissemination of these technologies into clinical practice has been challenging, similar to the challenges that face the widespread treatment of thoracoabdominal aneurysm in an open surgical manner.
Devices
Aortic Components
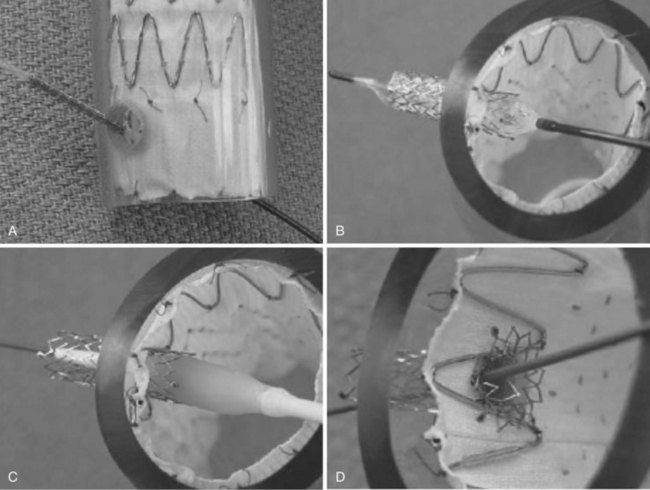
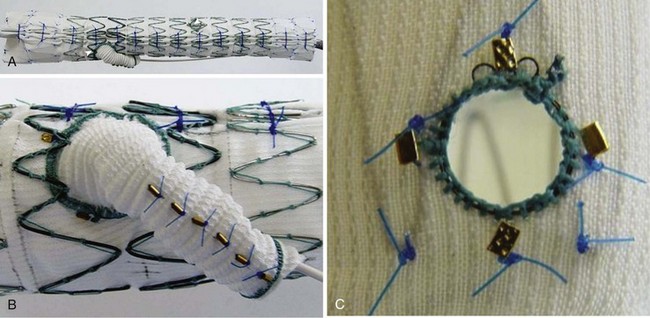
Fenestrated grafts are currently commercially available within the United States, and these are based on the Zenith platform. Other versions are currently available under clinical investigational trials. Fenestrations and side-arm branches are the two basic models used to make an array of thoracoabdominal devices, each of which has their own merits and limitations (Table 37-1).18 Fenestrations are circular or elliptical holes created in the graft fabric in a specific location, reinforced with a nitinol ring to provide a stable region against which to expand a balloon-mounted stent-graft (Figure 37-1). The fenestrations may be small (6 mm wide and 6 or 8 mm high, typically used for the renal arteries) or large (8 to 12 mm in diameter, usually used for visceral vessels; e.g., celiac and superior mesenteric artery). Large fenestrations may have a crossing strut or may be strut free depending on whether it is desired to place a mating stent-graft into the target branch vessel. Scallops are U-shaped openings at the ends of a stent-graft that may be used to accommodate branches when such branches are not directly involved in the aneurysm. Side-arm branches are most frequently used in the setting of larger TAAs (types II and III) and may be oriented axially (straight up and down) or angled (simple angle or helical wraps; Figure 37-2). Side-arm branches are most often directed in a caudal direction, but occasionally have been directed cranially. The branches may reside within (internal), exterior (external), or both (internal–external) to the aortic graft. Covered stents are coupled with branch devices to exclude the aneurysm from blood flow. A balloon-expandable stent-graft (Jomed [Abbott Park, Ill] or Atrium [Atrium USA, Hudson NH]) is used to create a seal between the aortic component and the target vessel when fenestrations are used. The proximal (aortic end) of such stents is flared against the nitinol ring attached circumferentially to the aortic fenestration to achieve a seal. In contrast, self-expanding stent-grafts (Fluency [Bard Medical, Covington, Ga] or Viabahn [W.L. Gore and Associates Ins, Flogstaff, Az]) are typically used with side-arm branches to better accommodate long distances, tortuosity, and achieve better overlap with the aortic device. Often, hybrid fenestrated or branched devices are used (ones that contain both fenestrations and side-arms) to better accommodate for specific types of anatomy (Figure 37-3).
TABLE 37-1 Advantages and Limitations of Devices Using Fenestrations and Side-Arm Branches for Excluding Thoracoabdominal Aortic Aneurysms
| Fenestrations | Side-Arm Branched | |
|---|---|---|
| Advantages | Less aortic coverage, thus minimizing paraplegia risk | Errors or design and deployment are better tolerated |
| Can be loaded into a low-profile sheath Can be easily angled in line with the native vessel geometry. side-arm branches impart a downward angle. This can place stress on the target vessel and bridging stent if the target vessel traverses upward and not downward. | Greater overlap between aortic component and mating stent graft | |
| Optimal for vessels oriented longitudinal to the aortic axis (such as superior mesenteric artery) Flexibility of self-expandable stent grafts reduces target artery distortion and damage More proximal aortic coverage required Profile is increased because of side arms Mating stent grafts, transcending through a distance of unsupported aneurysm, may link Aortic component must be tapered in small aortic lumens, thus decreasing the cross-sectional area and increasing the risk of late migration | ||
| Not prone to kinks | ||
| Limitations | Errors of design are not well tolerated | |
| Less sealing zone between the main aortic segment and mating stent graft | ||
| Balloon-expandable stent grafts are more prone to fracture in the arterial environment | ||
| Challenging to traverse long distances from the fenestration origin to the target branch vessel |
From Qureshi MA, Greenberg RK: Endovascular stent grafting for TAAA. In Franco KL, Thouraui VH, editors: Cardiothoracic surgery review, Philadelphia, 2011, Lippincott Williams Wilkins.
The devices may be customized to fit patient-specific anatomy or work “off-the-shelf ” when a standardized design allows for treatment of a subset of patients with common anatomy akin to branched grafts used to preserve internal iliac patency.19 The later types of devices rely on the concept that there is some consistency between aneurysm patients in regard to visceral branch morphology. However, the aortic diameter, and extent of the aneurysm vary in most patients, and thus patients considered for standardized devices must be lumped into groups (e.g., juxtarenal aneurysms, type IV thoracoabdominal aneurysm, type II and III thoracoabdominal aneurysms) to assess the proximal and distal extent of the required repair. Evaluation of preoperative images for patients intended for fenestrated stent-grafts demonstrated that several off-the-shelf models would be required to fit the majority of patients20; however, with modifications that provide a greater range of target vessels for individual fenestrations, it appears that only one or two visceral segment designs will be necessary to accommodate the majority of patients with juxtarenal and pararenal aneurysms.18
Preoperative Planning and Device Selection
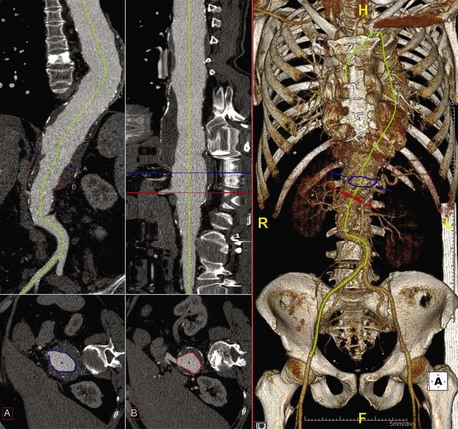
High-resolution computed tomography angiography (CTA) of the chest, abdomen, and pelvis is required with thin (z-plane) reconstructions. This must be accomplished with a contrast injection and a properly timed bolus to allow for opacification of the entire aorta and its branches. These images are then evaluated using a three-dimensional imaging program that allows for the creation of centerline-of-flow (CLF) reconstructions allowing for accurate and reproducible diameter measurements and the assessment of visceral branch relational morphology (Figure 37-4, see color plate). The CLF reconstructions are typically done in a semiautomated fashion, allowing the clinician to ensure the accuracy and modify the computed CLF to ensure accuracy specific to the repair strategy. Some companies offer these services via an internet network (or “cloud network”) in an effort to support the planning and sizing of the endovascular grafts. Regardless of the method used to obtain and process the imaging, the following pieces of information are critical to choose and design an appropriate device.
1. The proximal extent of the disease will determine the cranial extent of the repair and which vessels should be incorporated into the repair. All attempts should be made to extend the repair into healthy aorta regardless of the number of visceral vessels that are required to incorporate. One can assume that healthy aorta will be cylindrical, not excessively tortuous, have a length of 2 cm or more, and be generally free of debris; this will provide the stability for the repair in the long term. Aneurysms should be classified in accordance with the Society for Vascular Surgery (SVS) reporting standards classifications system.21
2. The proximal aortic diameter orthogonal to the CLF. Devices are then oversized 10% to 20% in comparison to the measured aortic diameter.
3. The geometric relationship between the visceral vessels (the longitudinal and radial location of each vessel intended to be incorporated into the repair) and the orientation of the visceral vessels to the aortic centerline is required to access eligibility to be treated with a standardized device, or to customize a fenestrated or branched device. This relationship (in conjunction with the luminal diameter around the visceral segment) will help to define whether fenestrations or branches should be used, how the fenestrations should be oriented, and whether branches should be oriented in an upward or downward fashion.
4. The location and morphology of the distal seal (i.e., distal aorta, old surgical graft, or iliac arteries).
5. The tortuosity of the arterial system. This will help to define an overall strategy for the repair, such as when visceral vessels should be accessed from above or below, from which side the main device should be introduced, and the orientation of a contralateral limb (if required).
In addition to these items, certain additional factors also must be considered. Thoracoabdominal aortic aneurysm (TAAA) can be extensive, with diseased aortic lengths in excess of 350 mm. The creation and deployment of such grafts creates many challenges; consequently, nearly all such repairs are modular with the longest graft components rarely longer than 270 mm. In such circumstances, the thoracic component of the aneurysm is repaired with conventional thoracic devices. The visceral segment is repaired with a fenestrated or branched device that is subsequently mated proximally to the thoracic device and distally to a bifurcated device when necessary. Attention must be directed to the overlap between modular components in regard to sealing and resistance to late separation. In general, when barbs are not used to secure joints between devices, a minimum of four stents of overlap is desired.22,23 The use of barbs that extend from the inner device to the outer device drops the required overlap length to two stents, provided that the overlap occurs in the straight portion of the aorta. Nearly all fenestrated and most branched devices taper to a distal diameter of 22 to 24 mm, allowing for mating with standard bifurcated components. Most commonly the tapers are created immediately below the lowest renal fenestration, allowing for large graft diameters in the region of the fenestrations to supplement seal and fixation. In the setting of side-arm branches, the tapers usually occur as the side-arms exit from the aortic lumen.
Standardized Visceral Segment Devices
Certain anatomic considerations must be evaluated when determining whether a customized device is required or a standard device may be used. In most cases, customized devices will more accurately fit the anatomy for which it was designed, unless the morphology of the patient conforms exactly to the geometry of the intended design. However, the extent to which the target vessels are offset from the design will have implications as to the technical challenges that may be encountered during deployment as well as the long-term integrity of the repair. Discrepancies between target vessel location and the location of aortic fenestrations must be accommodated for by the placement of mating stent-grafts that create a certain amount of stability, forcing the fenestration to align with the vessel and vice versa. Branches provide more leeway in both design and required alignment characteristics than fenestrations, but the mating stent-grafts still must bridge any cranial–caudal discrepancies as well as rotational angulation.24,25 An assessment of the acceptable mating stent-graft length that travels in an unsupported manner through the aneurysm must be made when considering patients for standardized side-arm branch designs. Most critically, standardized devices have an intended sealing location (i.e., below the kidneys, below the superior mesenteric artery, within the straight portion of the thoracic aorta, or the ascending aorta). It would be inadvisable to treat a patient with disease proximal to the intended sealing location with a standardized device if at all avoidable. Device manufacturers provide a range of morphologies that are acceptable for a standardized design, but at this point, none have published any sizable series, and no long-term results exist. The repercussions of late growth around the sealing and fixation portion of any repair involving aortic branches are considerable, making potential salvage of the endovascular device extremely complicated. Therefore, analogous to the use of infrarenal devices,26 it would be prudent to exercise caution when straying from the guidelines provided by manufacturers for fenestrated and branched devices.
Procedure
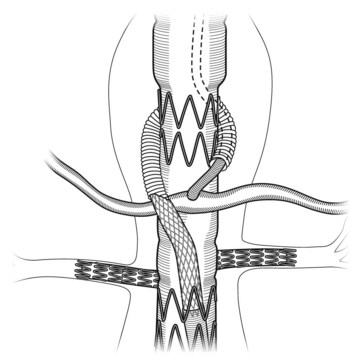
Implantation is typically performed using general endotracheal anesthesia, although regional anesthesia can be used for reasonably short cases. A lumbar drain is placed for drainage of cerebrospinal fluid when extensive coverage of the aorta is anticipated or interruption of contributing blood supply to the artery of Adamkiewicz (T8 to Ll) is coupled with another pathology (e.g., compromised internal iliac arteries or prior abdominal aortic aneurism [AAA] repair). Bilateral femoral arteries are accessed (via cutdown or percutaneously), and selective exposure of brachial or axillary artery access sites is performed (in case of side-arm branches). The primary device is delivered over a stiff wire that is usually placed into the ascending aorta. The device is then oriented appropriately and then unsheathed, with the fenestrations or side arms corresponding to their respective branch vessels. The sequences of mating the aortic component with the target vessels are detailed as follows. In general, each visceral branch is cannulated from within the aortic prosthesis. After access into each visceral vessel, the aortic component is allowed to completely expand by removing the constraining wires. Balloon expandable stent-grafts (fenestrations) or self-expanding stent-grafts (side-arm branches) are then delivered and deployed. Balloon-expandable stent-grafts are flared proximally. It is common to reinforce the lining of the self-expanding mating stent-grafts in some designs with supplemental stents to ensure the absence of kinking and provide stiffness to the system. The aortic delivery system is removed, and proximal thoracic, distal bifurcated, or internal iliac components are added as required to complete the repair. Repeated angiography is performed at the conclusion of the procedure to ensure effective sac exclusion, preserve essential vessels, and detect any endoleaks. Once sac exclusion is confirmed, the device sheath is removed, and arteriotomies are repaired. Procedural details have been outlined in other publications.27
Reinforced fenestrated branches:
1. Access primary aortic graft from contralateral groin.
2. Selectively cannulate the desired fenestration.
3. Establish 6- to 7-French sheath access into the target branch.
4. Deploy balloon-expandable stent-graft mounted on a balloon sized to the target vessel diameter.
5. Flare the aortic portion of the balloon-expandable stent-graft with a 10- to 12-mm balloon. Optionally, larger aortic balloons may be used.
1. Snare preloaded wire from remote access site (brachial or axillary).
2. Place a sheath (7 to 9 French) over preloaded wire.
3. Deploy primary aortic stent-graft after proper longitudinal and rotational alignment.
4. Advance sheath over preloaded wire into target directional branch.
5. Double puncture brachial–axillary sheath and advance hydrophilic catheter or wire into branch.
6. Cannulate target branch vessel and place stiff wire (Rosen wire).
7. Remove preloaded wire, thus losing all through-and-through access.
8. Advance self-expanding stent-graft into desired location and deploy with a minimum of 2 cm overlap into target vessel and 2 cm overlap into aortic endograft.
9. Selectively used additional stents or stent-grafts to ensure adequate overlap and an absence of kinking.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree